Distinct roles of Cdc42 in thymopoiesis and effector and memory T cell differentiation
- PMID: 21455314
- PMCID: PMC3063799
- DOI: 10.1371/journal.pone.0018002
Distinct roles of Cdc42 in thymopoiesis and effector and memory T cell differentiation
Abstract
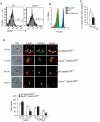
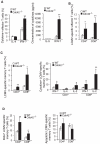
Cdc42 of the Rho GTPase family has been implicated in cell actin organization, proliferation, survival, and migration but its physiological role is likely cell-type specific. By a T cell-specific deletion of Cdc42 in mouse, we have recently shown that Cdc42 maintains naïve T cell homeostasis through promoting cell survival and suppressing T cell activation. Here we have further investigated the involvement of Cdc42 in multiple stages of T cell differentiation. We found that in Cdc42(-/-) thymus, positive selection of CD4(+)CD8(+) double-positive thymocytes was defective, CD4(+) and CD8(+) single-positive thymocytes were impaired in migration and showed an increase in cell apoptosis triggered by anti-CD3/-CD28 antibodies, and thymocytes were hyporesponsive to anti-CD3/-CD28-induced cell proliferation and hyperresponsive to anti-CD3/-CD28-stimulated MAP kinase activation. At the periphery, Cdc42-deficient naive T cells displayed an impaired actin polymerization and TCR clustering during the formation of mature immunological synapse, and showed an enhanced differentiation to Th1 and CD8(+) effector and memory cells in vitro and in vivo. Finally, Cdc42(-/-) mice exhibited exacerbated liver damage in an induced autoimmune disease model. Collectively, these data establish that Cdc42 is critically involved in thymopoiesis and plays a restrictive role in effector and memory T cell differentiation and autoimmunity.
Conflict of interest statement
Figures



Similar articles
-
The RhoA- and CDC42-specific exchange factor Dbs promotes expansion of immature thymocytes and deletion of double-positive and single-positive thymocytes.Eur J Immunol. 2004 Mar;34(3):806-816. doi: 10.1002/eji.200324400. Eur J Immunol. 2004. PMID: 14991610
-
CD28 ligation costimulates cell death but not maturation of double-positive thymocytes due to defective ERK MAPK signaling.J Immunol. 2006 Nov 1;177(9):6098-107. doi: 10.4049/jimmunol.177.9.6098. J Immunol. 2006. PMID: 17056536
-
Rho GTPase Cdc42 is essential for human T-cell development.Haematologica. 2010 Mar;95(3):367-75. doi: 10.3324/haematol.2009.006890. Haematologica. 2010. PMID: 20207844 Free PMC article.
-
The biology of recent thymic emigrants.Annu Rev Immunol. 2013;31:31-50. doi: 10.1146/annurev-immunol-032712-100010. Epub 2012 Nov 1. Annu Rev Immunol. 2013. PMID: 23121398 Review.
-
Positive and negative thymocyte selection.Crit Rev Immunol. 1998;18(4):359-70. doi: 10.1615/critrevimmunol.v18.i4.40. Crit Rev Immunol. 1998. PMID: 9704194 Review.
Cited by
-
Mevalonate metabolism-dependent protein geranylgeranylation regulates thymocyte egress.J Exp Med. 2020 Feb 3;217(2):e20190969. doi: 10.1084/jem.20190969. J Exp Med. 2020. PMID: 31722972 Free PMC article.
-
Cdc42 defect reveals insights into microvilli organization and function in T cell immunity.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2505291122. doi: 10.1073/pnas.2505291122. Epub 2025 Jul 25. Proc Natl Acad Sci U S A. 2025. PMID: 40711916 Free PMC article.
-
Serum cell division cycle 42 reflects the treatment response and survival in patients with advanced cervical cancer who receive immune checkpoint inhibitor treatment.Oncol Lett. 2023 Aug 8;26(3):414. doi: 10.3892/ol.2023.14000. eCollection 2023 Sep. Oncol Lett. 2023. PMID: 37600330 Free PMC article.
-
The relation of blood cell division control protein 42 level with disease risk, comorbidity, tumor features/markers, and prognosis in colorectal cancer patients.J Clin Lab Anal. 2022 Jul;36(7):e24572. doi: 10.1002/jcla.24572. Epub 2022 Jun 23. J Clin Lab Anal. 2022. PMID: 35735582 Free PMC article.
-
Gene targeting RhoA reveals its essential role in coordinating mitochondrial function and thymocyte development.J Immunol. 2014 Dec 15;193(12):5973-82. doi: 10.4049/jimmunol.1400839. Epub 2014 Nov 14. J Immunol. 2014. PMID: 25398325 Free PMC article.
References
-
- Bowen H, Kelly A, Lee T, Lavender P. Control of cytokine gene transcription in Th1 and Th2 cells. Clin Exp Allergy. 2008;38:1422–1431. - PubMed
-
- Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol. 2010;22:303–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

