Secretory phospholipase A₂ responsive liposomes
- PMID: 21455978
- PMCID: PMC3196631
- DOI: 10.1002/jps.22530
Secretory phospholipase A₂ responsive liposomes
Abstract
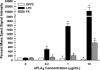
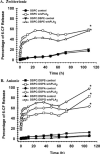
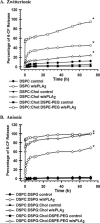
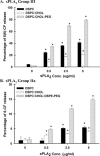
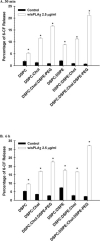
Secretory phospholipase A(2) (sPLA(2)) expression is increased in several cancers and has been shown to trigger release from some lipid carriers. This study used electrospray ionization mass spectrometry (ESI-MS) and release of 6-carboxyfluorescein (6-CF) to determine the effects of sPLA(2) on various liposome formulations. Different combinations of zwitterionic [1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine, 1,2-distearoyl-sn-glycero-3-phosphatidylcholine, and 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine (DSPE)] and anionic [1,2-distearoyl-sn-glycero-3-phosphatidic acid, 1,2-distearoyl-sn-glycero-3-phosphatidylglycerol (DSPG), 1,2-distearoyl-sn-glycero-3-phosphatidylserine, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-poly(ethylene glycol) 2000 (DSPE-PEG)] phospholipids were examined. DSPG and DSPE were most susceptible to sPLA(2)-mediated degradation compared with other phospholipids. Increased 6-CF release was observed after inclusion of 10 mol % DSPE and anionic lipids into different liposome formulations. Group IIa sPLA(2)-mediated 6-CF release was less than Group III and relatively insensitive to cholesterol (Chol), whereas Chol reduced sPLA(2)-mediated release. Inclusion of DSPE-PEG increased sPLA(2)-mediated 6-CF release, whereas serum reduced lipid degradation and 6-CF release significantly. These data demonstrate that ESI-MS and 6-CF release were useful in determining the selectivity of sPLA(2) and release from liposomes, that differences in the activity of different sPLA(2) isoforms exist, and that DSPE-PEG enhanced sPLA(2)-mediated release of liposomal constituents. These findings will aid in the selection of lipids and optimization of the kinetics of drug release for the treatment of cancers and diseases of inflammation in which sPLA(2) expression is increased.
Copyright © 2011 Wiley-Liss, Inc.
Figures


References
-
- Gabizon A. Liposomes as a drug delivery system in cancer chemotherapy. Horiz Biochem Biophys. 1989;9:185–211. - PubMed
-
- Muggia FM. Liposomal encapsulated anthracyclines: new therapeutic horizons. Current Oncol. 2001;3:156–162. - PubMed
-
- Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta. 1991;1070(1):187–192. - PubMed
-
- Sharma US, Sharma A, Chau RI, Straubinger RM. Liposome-mediated therapy of intracranial brain tumors in a rat model. Pharm Res. 1997;14(8):992–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

