Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy
- PMID: 21455997
- PMCID: PMC3908827
- DOI: 10.1002/cne.22623
Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy
Abstract
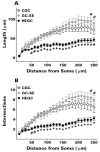
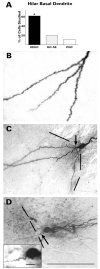
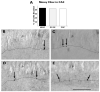
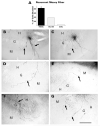
After pilocarpine-induced status epilepticus, many granule cells born into the postseizure environment migrate aberrantly into the dentate hilus. Hilar ectopic granule cells (HEGCs) are hyperexcitable and may therefore increase circuit excitability. This study determined the distribution of their axons and dendrites. HEGCs and normotopic granule cells were filled with biocytin during whole-cell patch clamp recording in hippocampal slices from pilocarpine-treated rats. The apical dendrite of 86% of the biocytin-labeled HEGCs extended to the outer edge of the dentate molecular layer. The total length and branching of HEGC apical dendrites that penetrated the molecular layer were significantly reduced compared with apical dendrites of normotopic granule cells. HEGCs were much more likely to have a hilar basal dendrite than normotopic granule cells. They were about as likely as normotopic granule cells to project to CA3 pyramidal cells within the slice, but were much more likely to send at least one recurrent mossy fiber into the molecular layer. HEGCs with burst capability had less well-branched apical dendrites than nonbursting HEGCs, their dendrites were more likely to be confined to the hilus, and some exhibited dendritic features similar to those of immature granule cells. HEGCs thus have many paths along which to receive synchronized activity from normotopic granule cells and to transmit their own hyperactivity to both normotopic granule cells and CA3 pyramidal cells. They may therefore contribute to the highly interconnected granule cell hubs that have been proposed as crucial to development of a hyperexcitable, potentially seizure-prone circuit.
Copyright © 2011 Wiley-Liss, Inc.
Figures



Similar articles
-
High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats.J Neurophysiol. 2010 Dec;104(6):3293-304. doi: 10.1152/jn.00663.2010. Epub 2010 Sep 29. J Neurophysiol. 2010. PMID: 20881195 Free PMC article.
-
Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry.J Comp Neurol. 2000 Dec 11;428(2):240-53. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. J Comp Neurol. 2000. PMID: 11064364
-
New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction.Epilepsia. 2012 Jun;53 Suppl 1(0 1):109-15. doi: 10.1111/j.1528-1167.2012.03480.x. Epilepsia. 2012. PMID: 22612815 Free PMC article.
-
Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy.Epilepsia. 2012 Jun;53 Suppl 1:98-108. doi: 10.1111/j.1528-1167.2012.03481.x. Epilepsia. 2012. PMID: 22612814 Review.
-
Neuroplasticity in the damaged dentate gyrus of the epileptic brain.Prog Brain Res. 2002;136:319-28. doi: 10.1016/s0079-6123(02)36027-8. Prog Brain Res. 2002. PMID: 12143392 Review.
Cited by
-
PTEN deletion increases hippocampal granule cell excitability in male and female mice.Neurobiol Dis. 2017 Dec;108:339-351. doi: 10.1016/j.nbd.2017.08.014. Epub 2017 Sep 21. Neurobiol Dis. 2017. PMID: 28855130 Free PMC article.
-
Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy.J Neurosci. 2012 Jan 25;32(4):1183-96. doi: 10.1523/JNEUROSCI.5342-11.2012. J Neurosci. 2012. PMID: 22279204 Free PMC article.
-
PTEN deletion from adult-generated dentate granule cells disrupts granule cell mossy fiber axon structure.Neurobiol Dis. 2015 Mar;75:142-50. doi: 10.1016/j.nbd.2014.12.029. Epub 2015 Jan 17. Neurobiol Dis. 2015. PMID: 25600212 Free PMC article.
-
Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity.J Neurosci. 2013 May 22;33(21):8926-36. doi: 10.1523/JNEUROSCI.5161-12.2013. J Neurosci. 2013. PMID: 23699504 Free PMC article.
-
Disrupted hippocampal network physiology following PTEN deletion from newborn dentate granule cells.Neurobiol Dis. 2016 Dec;96:105-114. doi: 10.1016/j.nbd.2016.09.004. Epub 2016 Sep 3. Neurobiol Dis. 2016. PMID: 27597527 Free PMC article.
References
-
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult dentate gyrus. Brain Res. 2004;1017:21–31. - PubMed
-
- Bonde S, Ekdahl CT, Lindvall O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci. 2006;23:965–974. - PubMed
-
- Bough KJ, Mott DD, Dingledine RJ. Medial perforant path inhibition mediated by mGluR7 is reduced after status epilepticus. J Neurophysiol. 2004;92:1549–1557. - PubMed
-
- Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophysiol. 1999;81:712–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

