Regression of prostate tumors upon combination of hormone ablation therapy and celecoxib in vivo
- PMID: 21456064
- PMCID: PMC3139688
- DOI: 10.1002/pros.21297
Regression of prostate tumors upon combination of hormone ablation therapy and celecoxib in vivo
Abstract
Background: Hormonal ablation is the standard of treatment for advanced androgen-dependent prostate cancer. Although tumor regression is usually achieved at first, the cancer inevitably evolves toward androgen-independence, in part because of the development of mechanisms of resistance and in part because at the tissue level androgen withdrawal is not fully attained. Current research efforts are focused on new therapeutic strategies that will increase the effectiveness of androgen withdrawal and delay recurrence. We used a syngeneic pseudo-orthotropic mouse model of prostate cancer to test the efficacy of combining androgen withdrawal with FDA-approved COX-2 inhibitor celecoxib.
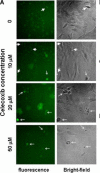
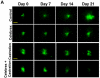
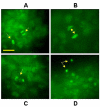
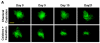
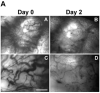
Methods: GFP-tagged TRAMP-C2 cells were co-implanted with prostate tissue in the dorsal chamber model and tumors were allowed to establish and vascularize. Tumor growth and angiogenesis were monitored in real-time using fluorescent intravital microscopy (IVM). Androgen withdrawal in mice was achieved using surgical castration or chemical hormonal ablation, alone or in combination with celecoxib (15 mg/kg, twice daily).
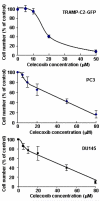
Results: Celecoxib alone decreased the growth of prostate tumors mostly by inducing mitotic failure, which resulted in increased apoptosis. Surprisingly, celecoxib did not possess significant angiostatic activity. Surgical or chemical castration prevented the growth of prostate tumors and this, on the other hand, was associated with disruption of the tumor vasculature. Finally, androgen withdrawal combined with celecoxib caused tumor regression through decreased angiogenesis and increased mitosis arrest and apoptosis.
Conclusion: Celecoxib, a relatively safe COX-2-selective anti-inflammatory drug, significantly increases the efficacy of androgen withdrawal in vivo and warrants further investigation as a complement therapy for advanced prostate cancer.
Copyright © 2010 Wiley-Liss, Inc.
Figures













References
-
- Stock D, Groome PA, Siemens DR. Inflammation and prostate cancer: a future target for prevention and therapy? The Urologic clinics of North America. 2008;35(1):117–130. vii. - PubMed
-
- Furstenberger G, Krieg P, Muller-Decker K, Habenicht AJ. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis? International journal of cancer. 2006;119(10):2247–2254. - PubMed
-
- Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17(2):55–67. - PubMed
-
- Sahin M, Sahin E, Gumuslu S. Cyclooxygenase-2 in cancer and angiogenesis. Angiology. 2009;60(2):242–253. - PubMed
-
- Pruthi RS, Wallen EM. Cyclooxygenase-2: a therapeutic target for prostate cancer. Clinical genitourinary cancer. 2005;4(3):203–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

