DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes
- PMID: 21456541
- PMCID: PMC3118939
- DOI: 10.1021/tx200091y
DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes
Abstract
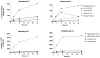
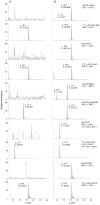
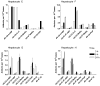
DNA adduct formation of the aromatic amine, 4-aminobiphenyl (4-ABP), a known human carcinogen present in tobacco smoke, and the heterocyclic aromatic amines (HAAs), 2-amino-9H-pyrido[2,3-b]indole (AαC), 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), and 2-amino-3,8-dimethylmidazo[4,5-f]quinoxaline (MeIQx), potential human carcinogens, which are also present in tobacco smoke or formed during the high-temperature cooking of meats, was investigated in freshly cultured human hepatocytes. The carcinogens (10 μM) were incubated with hepatocytes derived from eight different donors for time periods up to 24 h. The DNA adducts were quantified by liquid chromatography-electrospray ionization mass spectrometry with a linear quadrupole ion trap mass spectrometer. The principal DNA adducts formed for all of the carcinogens were N-(deoxyguanosin-8-yl) (dG-C8) adducts. The levels of adducts ranged from 3.4 to 140 adducts per 10(7) DNA bases. The highest level of adduct formation occurred with AαC, followed by 4-ABP, then by PhIP, MeIQx, and IQ. Human hepatocytes formed dG-C8-HAA-adducts at levels that were up to 100-fold greater than the amounts of adducts produced in rat hepatocytes. In contrast to HAA adducts, the levels of dG-C8-4-ABP adduct formation were similar in human and rat hepatocytes. These DNA binding data demonstrate that the rat, an animal model that is used for carcinogenesis bioassays, significantly underestimates the potential hepatic genotoxicity of HAAs in humans. The high level of DNA adducts formed by AαC, a carcinogen produced in tobacco smoke at levels that are up to 100-fold higher than the amounts of 4-ABP, is noteworthy. The possible causal role of AαC in tobacco-associated cancers warrants investigation.
Figures

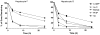


References
-
- Clayson DB. Specific aromatic amines as occupational bladder carcinogens. Natl Cancer Inst Monogr. 1981:15–19. - PubMed
-
- IARC. Tobacco smoking. Vol. 38. International Agency for Research on Cancer; 1986. Monographs on the Evaluation of Carcinogenic Risks to Humans.
-
- International Agency for Research on Cancer. Overall evaluation of carcinogenicity: An updating of IARC monographs 1–42. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 1987;7(suppl) - PubMed
-
- Stavric B, Klassen R, Miles W. Gas-liquid chromatographic-mass spectrometric determination of alpha- and beta-naphthylamines in FD&C Red No. 2 (amaranth) J Assoc Off Anal Chem. 1979;62:1020–1026. - PubMed
-
- Davis VM, Bailey JE., Jr Chemical reduction of FD&C yellow No. 5 to determine combined benzidine. J Chromatogr. 1993;635:160–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

