The cognitive neuroscience of human memory since H.M
- PMID: 21456960
- PMCID: PMC3192650
- DOI: 10.1146/annurev-neuro-061010-113720
The cognitive neuroscience of human memory since H.M
Abstract
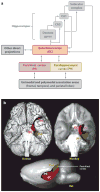
Work with patient H.M., beginning in the 1950s, established key principles about the organization of memory that inspired decades of experimental work. Since H.M., the study of human memory and its disorders has continued to yield new insights and to improve understanding of the structure and organization of memory. Here we review this work with emphasis on the neuroanatomy of medial temporal lobe and diencephalic structures important for memory, multiple memory systems, visual perception, immediate memory, memory consolidation, the locus of long-term memory storage, the concepts of recollection and familiarity, and the question of how different medial temporal lobe structures may contribute differently to memory functions.
Figures




References
-
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:810–23. - PubMed
-
- Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Suppes P, editors. Contemporary Developments in Mathematical Psychology. San Francisco: Freeman; 1974. pp. 243–90.
-
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–39. - PubMed
-
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–74. - PubMed
-
- Barton JJS, Cherkasova M. Face imagery and its relation to perception and covert recognition in prosopagnosia. Neurology. 2003;61:220–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

