Amyloid precursor protein processing and Alzheimer's disease
- PMID: 21456963
- PMCID: PMC3174086
- DOI: 10.1146/annurev-neuro-061010-113613
Amyloid precursor protein processing and Alzheimer's disease
Abstract
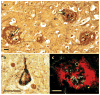
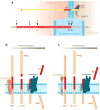
Alzheimer's disease (AD), the leading cause of dementia worldwide, is characterized by the accumulation of the β-amyloid peptide (Aβ) within the brain along with hyperphosphorylated and cleaved forms of the microtubule-associated protein tau. Genetic, biochemical, and behavioral research suggest that physiologic generation of the neurotoxic Aβ peptide from sequential amyloid precursor protein (APP) proteolysis is the crucial step in the development of AD. APP is a single-pass transmembrane protein expressed at high levels in the brain and metabolized in a rapid and highly complex fashion by a series of sequential proteases, including the intramembranous γ-secretase complex, which also process other key regulatory molecules. Why Aβ accumulates in the brains of elderly individuals is unclear but could relate to changes in APP metabolism or Aβ elimination. Lessons learned from biochemical and genetic studies of APP processing will be crucial to the development of therapeutic targets to treat AD.
Figures


References
-
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–76. - PubMed
-
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–31. - PubMed
-
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem. 2001;276:40353–61. - PubMed
-
- Asai M, Hattori C, Szabo B, Sasagawa N, Maruyama K, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun. 2003;301:231–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

