Amyloid structure: conformational diversity and consequences
- PMID: 21456964
- PMCID: PMC3817101
- DOI: 10.1146/annurev-biochem-090908-120656
Amyloid structure: conformational diversity and consequences
Abstract
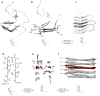
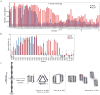
Many, perhaps most, proteins, are capable of forming self-propagating, β-sheet (amyloid) aggregates. Amyloid-like aggregates are found in a wide range of diseases and underlie prion-based inheritance. Despite intense interest in amyloids, structural details have only recently begun to be revealed as advances in biophysical approaches, such as hydrogen-deuterium exchange, X-ray crystallography, solid-state nuclear magnetic resonance (SSNMR), and cryoelectron microscopy (cryoEM), have enabled high-resolution insights into their molecular organization. Initial studies found that despite the highly divergent primary structure of different amyloid-forming proteins, amyloids from different sources share many structural similarities. With higher-resolution information, however, it has become clear that, on the molecular level, amyloids comprise a wide diversity of structures. Particularly surprising has been the finding that identical polypeptides can fold into multiple, distinct amyloid conformations and that this structural diversity can lead to distinct heritable prion states or strains.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

