Mechanistic links between oxidative stress and disuse muscle atrophy
- PMID: 21457104
- PMCID: PMC3208252
- DOI: 10.1089/ars.2011.3973
Mechanistic links between oxidative stress and disuse muscle atrophy
Abstract
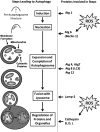
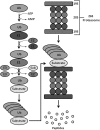
Long periods of skeletal muscle inactivity promote a loss of muscle protein resulting in fiber atrophy. This disuse-induced muscle atrophy results from decreased protein synthesis and increased protein degradation. Recent studies have increased our insight into this complicated process, and evidence indicates that disturbed redox signaling is an important regulator of cell signaling pathways that control both protein synthesis and proteolysis in skeletal muscle. The objective of this review is to outline the role that reactive oxygen species play in the regulation of inactivity-induced skeletal muscle atrophy. Specifically, this report will provide an overview of experimental models used to investigate disuse muscle atrophy and will also highlight the intracellular sources of reactive oxygen species and reactive nitrogen species in inactive skeletal muscle. We then will provide a detailed discussion of the evidence that links oxidants to the cell signaling pathways that control both protein synthesis and degradation. Finally, by presenting unresolved issues related to oxidative stress and muscle atrophy, we hope that this review will serve as a stimulus for new research in this exciting field.
Figures




References
-
- Adams V. Yu J. Mobius-Winkler S. Linke A. Weigl C. Hilbrich L. Schuler G. Hambrecht R. Increased inducible nitric oxide synthase in skeletal muscle biopsies from patients with chronic heart failure. Biochem Mol Med. 1997;61:152–160. - PubMed
-
- Alirezaei M. Marin P. Nairn AC. Glowinski J. Premont J. Inhibition of protein synthesis in cortical neurons during exposure to hydrogen peroxide. J Neurochem. 2001;76:1080–1088. - PubMed
-
- Appell HJ. Duarte JA. Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–160. - PubMed
-
- Aucello M. Dobrowolny G. Musaro A. Localized accumulation of oxidative stress causes muscle atrophy through activation of an autophagic pathway. Autophagy. 2009;5:527–529. - PubMed
-
- Bechet D. Tassa A. Taillandier D. Combaret L. Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol. 2005;37:2098–2114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

