Birth of piglets from in vitro-produced, zona-intact porcine embryos vitrified in a closed system
- PMID: 21458047
- PMCID: PMC3115500
- DOI: 10.1016/j.theriogenology.2011.02.004
Birth of piglets from in vitro-produced, zona-intact porcine embryos vitrified in a closed system
Abstract
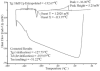
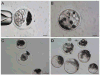
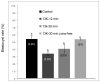
As the importance of swine models in biomedical research increases, it is essential to develop low-cost, high-throughput systems to cryopreserve swine germplasm for maintenance of these models. However, porcine embryos are exceedingly sensitive to low temperature and successful cryopreservation is generally limited to the use of vitrification in open systems that allow direct contact of the embryos with liquid nitrogen (LN(2)). This creates a high risk of pathogen transmission. Therefore, cryopreservation of porcine embryos in a "closed" system is of very high importance. In this study, in vitro-produced (IVP) porcine embryos were used to investigate cryosurvival and developmental potential of embryos cryopreserved in a closed system. Optimal centrifugal forces to completely disassociate intracellular lipids from blastomeres were investigated using Day-4 embryos. Cryosurvival of delipidated embryos was investigated by vitrifying the embryos immediately after centrifugation, or after development to blastocysts. In this study, centrifugation for 30 min at 13,000 g was adequate to completely delipidate the embryos; furthermore, these embryos were able to survive cryopreservation at a rate comparable to those centrifuged for only 12 min. When delipidated embryos were vitrified at the blastocyst stage, there was no difference in survival between embryos vitrified using OPS and 0.25 mL straws. Some embryos vitrified by each method developed to term. These experiments demonstrated that porcine embryos can be cryopreserved in a closed system after externalizing their intracellular lipids. This has important implications for banking swine models of human health and disease.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Mazur P, Leibo SP, Seidel GE., Jr Cryopreservation of the germplasm of animals used in biological and medical research: importance, impact, status, and future directions. Biol Reprod. 2008;78:2–12. - PubMed
-
- Prather RS, Hawley RJ, Carter DB, Lai L, Greenstein JL. Transgenic swine for biomedicine and agriculture. Theriogenology. 2003;59:115–23. - PubMed
-
- Critser JK, Laughlin MH, Prather RS, Riley LK. Proceedings of the Conference on Swine in Biomedical Research. ILAR J. 2009;50:89–94. - PubMed
-
- Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;46:146–52. - PubMed
-
- Fountain D, Ralston M, Higgins N, Gorlin JB, Uhl L, Wheeler C, Antin JH, Churchill WH, Benjamin RJ. Liquid nitrogen freezers: a potential source of microbial contamination of hematopoietic stem cell components. Transfusion. 1997;37:585–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

