Endoplasmic reticulum stress and pancreatic β-cell death
- PMID: 21458293
- PMCID: PMC3130122
- DOI: 10.1016/j.tem.2011.02.008
Endoplasmic reticulum stress and pancreatic β-cell death
Abstract
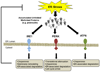
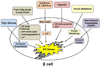
In pancreatic β-cells, the endoplasmic reticulum (ER) is an important cellular compartment for insulin biosynthesis, which accounts for half of the total protein production in these cells. Protein flux through the ER must be carefully monitored to prevent dysregulation of ER homeostasis and stress. ER stress elicits a signaling cascade known as the unfolded protein response (UPR), which influences both life and death decisions in cells. β-cell loss is a pathological component of both type 1 and type 2 diabetes, and recent findings suggest that ER stress is involved. In this review, we address the transition from the physiological ER stress response to the pathological response, and explore the mechanisms of ER stress-mediated β-cell loss during the progression of diabetes.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Melloul D, et al. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. - PubMed
-
- Goodge KA, Hutton JC. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic beta-cell. Semin Cell Dev Biol. 2000;11:235–242. - PubMed
-
- Harding HP, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Molecular Cell. 2001;7:1153–1163. - PubMed
-
- Scheuner D, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

