Structure of a domain-swapped FOXP3 dimer on DNA and its function in regulatory T cells
- PMID: 21458306
- PMCID: PMC3085397
- DOI: 10.1016/j.immuni.2011.02.017
Structure of a domain-swapped FOXP3 dimer on DNA and its function in regulatory T cells
Erratum in
- Immunity. 2011 Apr 22;34(4):627
Abstract
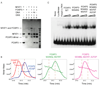
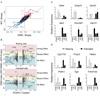
The transcription factor FOXP3 is essential for the suppressive function of regulatory T cells that are required for maintaining self-tolerance. We have solved the crystal structure of the FOXP3 forkhead domain as a ternary complex with the DNA-binding domain of the transcription factor NFAT1 and a DNA oligonucleotide from the interleukin-2 promoter. A striking feature of this structure is that FOXP3 forms a domain-swapped dimer that bridges two molecules of DNA. Structure-guided or autoimmune disease (IPEX)-associated mutations in the domain-swap interface diminished dimer formation by the FOXP3 forkhead domain without compromising FOXP3 DNA binding. These mutations also eliminated T cell-suppressive activity conferred by FOXP3, both in vitro and in a murine model of autoimmune diabetes in vivo. We conclude that FOXP3-mediated suppressor function requires dimerization through the forkhead domain and that mutations in the dimer interface can lead to the systemic autoimmunity observed in IPEX patients.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. - PubMed
-
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI044432/AI/NIAID NIH HHS/United States
- R01 DK070055/DK/NIDDK NIH HHS/United States
- AI40127/AI/NIAID NIH HHS/United States
- CA42471/CA/NCI NIH HHS/United States
- R01 AI048213/AI/NIAID NIH HHS/United States
- R01 GM077320/GM/NIGMS NIH HHS/United States
- GM064642/GM/NIGMS NIH HHS/United States
- R37 CA042471/CA/NCI NIH HHS/United States
- RC1 DA028422/DA/NIDA NIH HHS/United States
- R01 GM064642/GM/NIGMS NIH HHS/United States
- AI48213/AI/NIAID NIH HHS/United States
- R01 AI040127/AI/NIAID NIH HHS/United States
- R01 DK059279/DK/NIDDK NIH HHS/United States
- U01 HL100001/HL/NHLBI NIH HHS/United States
- RC2 HL102815/HL/NHLBI NIH HHS/United States
- R01 CA042471/CA/NCI NIH HHS/United States
- AI44432/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

