Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search
- PMID: 21458672
- PMCID: PMC3073827
- DOI: 10.1016/j.cell.2011.02.008
Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search
Abstract
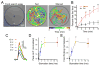
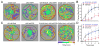
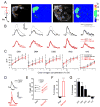
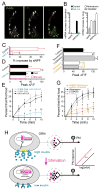
Internal physiological states influence behavioral decisions. We have investigated the underlying cellular and molecular mechanisms at the first olfactory synapse for starvation modulation of food-search behavior in Drosophila. We found that a local signal by short neuropeptide F (sNPF) and a global metabolic cue by insulin are integrated at specific odorant receptor neurons (ORNs) to modulate olfactory sensitivity. Results from two-photon calcium imaging show that starvation increases presynaptic activity via intraglomerular sNPF signaling. Expression of sNPF and its receptor (sNPFR1) in Or42b neurons is necessary for starvation-induced food-search behavior. Presynaptic facilitation in Or42b neurons is sufficient to mimic starvation-like behavior in fed flies. Furthermore, starvation elevates the transcription level of sNPFR1 but not that of sNPF, and insulin signaling suppresses sNPFR1 expression. Thus, starvation increases expression of sNPFR1 to change the odor map, resulting in more robust food-search behavior.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Hungry flies tune to vinegar.Cell. 2011 Apr 1;145(1):17-8. doi: 10.1016/j.cell.2011.03.018. Cell. 2011. PMID: 21458663 Free PMC article.
References
-
- Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600. - PubMed
-
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. - PubMed
-
- Brown MR, Crim JW, Arata RC, Cai HN, Chun C, Shen P. Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides. 1999;20:1035–1042. - PubMed
-
- Carlsson MA, Diesner M, Schachtner J, Nassel DR. Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J Comp Neurol. 2010;518:3359–3380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

