Thermodynamic analysis of mutant lac repressors
- PMID: 21459098
- PMCID: PMC4225698
- DOI: 10.1016/j.jmb.2011.03.057
Thermodynamic analysis of mutant lac repressors
Abstract
The lactose (lac) repressor is an allosteric protein that can respond to environmental changes. Mutations introduced into the DNA binding domain and the effector binding pocket affect the repressor's ability to respond to its environment. We have demonstrated how the observed phenotype is a consequence of altering the thermodynamic equilibrium constants. We discuss mutant repressors, which (1) show tighter repression; (2) induce with a previously noninducing species, orthonitrophenyl-β-D-galactoside; and (3) transform an inducible switch to one that is corepressed. The ability of point mutations to change multiple thermodynamic constants, and hence drastically alter the repressor's phenotype, shows how allosteric proteins can perform a wide array of similar yet distinct functions such as that exhibited in the Lac/Gal family of bacterial repressors.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
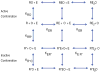
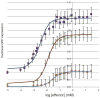

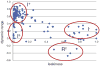
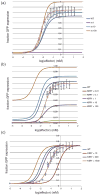
References
-
- Monod J, Changeux JP, Jacob F. Allosteric proteins and cellular control systems. J Mol Biol. 1963;6:306–329. - PubMed
-
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. - PubMed
-
- Jobe A, Bourgeois S. Lac repressor–operator interaction: VIII. Lactose is an anti-inducer of the lac operon. J Mol Biol. 1973;75:303–313. - PubMed
-
- Lewis M, Chang G, Horton NC, Kercher MA, Pace HC, Schumacher MA, et al. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. see comment. - PubMed
-
- Boelens R, Scheek RM, van Boom JH, Kaptein R. Complex of lac repressor headpiece with a 14 base-pair lac operator fragment studied by twodimensional nuclear magnetic resonance. J Mol Biol. 1987;193:213–216. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

