Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema
- PMID: 21459214
- PMCID: PMC3096445
- DOI: 10.1016/j.ophtha.2010.12.033
Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema
Abstract
Objective: To report expanded 2-year follow-up of a previously reported randomized trial evaluating intravitreal 0.5 mg ranibizumab or 4 mg triamcinolone combined with focal/grid laser compared with focal/grid laser alone for treatment of diabetic macular edema (DME).
Design: Multicenter, randomized clinical trial.
Participants: A total of 854 study eyes of 691 participants with visual acuity of 20/32 to 20/320 (approximate Snellen equivalent) and DME involving the fovea.
Methods: Continuation of procedures previously reported for the randomized trial.
Main outcome measures: Best-corrected visual acuity and safety at the 2-year visit.
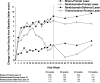
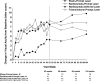
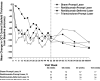
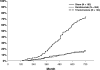
Results: At the 2-year visit, compared with the sham + prompt laser group, the mean change in the visual acuity letter score from baseline was 3.7 letters greater in the ranibizumab + prompt laser group (95% confidence interval adjusted for multiple comparisons [aCI], -0.4 to +7.7), 5.8 letters greater in the ranibizumab + deferred laser group (95% aCI, +1.9 to +9.8), and 1.5 letters worse in the triamcinolone + prompt laser group (95% aCI, -5.5 to +2.4). After the 1- to 2-year visit in the ranibizumab + prompt or deferred laser groups, the median numbers of injections were 2 and 3 (potential maximum of 13), respectively. At the 2-year visit, the percentages of eyes with central subfield thickness ≥250 μm were 59% in the sham + prompt laser group, 43% in the ranibizumab + prompt laser group, 42% in the ranibizumab + deferred laser group, and 52% in the triamcinolone + prompt laser group. No systemic events attributable to study treatment were apparent. Three eyes in 3 (0.8%) of 375 participants had injection-related endophthalmitis in the ranibizumab groups, whereas elevated intraocular pressure and cataract surgery were more frequent in the triamcinolone + prompt laser group.
Conclusions: The expanded 2-year results reported are similar to results published previously and reinforce the conclusions originally reported: Ranibizumab should be considered for patients with DME and characteristics similar to those of the cohort in this clinical trial, including vision impairment with DME involving the center of the macula.
Copyright © 2011 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study): 12-month data: report 2. Ophthalmology. 2010;117:1078–86. - PubMed
-
- U.S. National Institutes of Health ClinicalTrials.gov [January 5, 2010];Efficacy and safety of ranibizumab (intravitreal injections) in patients with visual impairment due to diabetic macular edema (RESTORE) ClinicalTrials.gov identifer NCT00687804. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00687804?term=nct00687804.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

