PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation
- PMID: 21459330
- PMCID: PMC3086520
- DOI: 10.1016/j.cmet.2011.03.004
PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation
Abstract
SIRT1 regulates energy homeostasis by controlling the acetylation status and activity of a number of enzymes and transcriptional regulators. The fact that NAD(+) levels control SIRT1 activity confers a hypothetical basis for the design of new strategies to activate SIRT1 by increasing NAD(+) availability. Here we show that the deletion of the poly(ADP-ribose) polymerase-1 (PARP-1) gene, encoding a major NAD(+)-consuming enzyme, increases NAD(+) content and SIRT1 activity in brown adipose tissue and muscle. PARP-1(-/-) mice phenocopied many aspects of SIRT1 activation, such as a higher mitochondrial content, increased energy expenditure, and protection against metabolic disease. Also, the pharmacologic inhibition of PARP in vitro and in vivo increased NAD(+) content and SIRT1 activity and enhanced oxidative metabolism. These data show how PARP-1 inhibition has strong metabolic implications through the modulation of SIRT1 activity, a property that could be useful in the management not only of metabolic diseases, but also of cancer.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

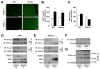
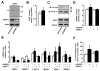

Comment in
-
A one and a two … expanding roles for poly(ADP-ribose) polymerases in metabolism.Cell Metab. 2011 Apr 6;13(4):353-355. doi: 10.1016/j.cmet.2011.03.011. Cell Metab. 2011. PMID: 21459317 Free PMC article.
References
-
- Adamietz P. Poly(ADP-ribose) synthase is the major endogenous nonhistone acceptor for poly(ADP-ribose) in alkylated rat hepatoma cells. Eur J Biochem. 1987;169:365–372. - PubMed
-
- Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun. 2006;349:353–359. - PubMed
-
- Allinson SL, Dianova II, Dianov GL. Poly(ADP-ribose) polymerase in base excision repair: always engaged, but not essential for DNA damage processing. Acta Biochim Pol. 2003;50:169–179. - PubMed
-
- Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

