Regulation of neural process growth, elaboration and structural plasticity by NF-κB
- PMID: 21459462
- PMCID: PMC3115056
- DOI: 10.1016/j.tins.2011.03.001
Regulation of neural process growth, elaboration and structural plasticity by NF-κB
Abstract
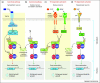
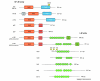
The nuclear factor-kappa B (NF-κB) family of transcription factors has recently emerged as a major regulator of the growth and elaboration of neural processes. NF-κB signaling has been implicated in controlling axon initiation, elongation, guidance and branching and in regulating dendrite arbor size and complexity during development and dendritic spine density in the adult. NF-κB is activated by a variety of extracellular signals, and either promotes or inhibits growth depending on the phosphorylation status of the p65 NF-κB subunit. These novel roles for NF-κB, together with recent evidence implicating NF-κB in the regulation of neurogenesis in the embryo and adult, have important implications for neural development and for learning and memory in the mature nervous system.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis.J Neurosci. 2011 Apr 6;31(14):5414-25. doi: 10.1523/JNEUROSCI.2456-10.2011. J Neurosci. 2011. PMID: 21471377 Free PMC article.
-
Targeting of NF-κB to Dendritic Spines Is Required for Synaptic Signaling and Spine Development.J Neurosci. 2018 Apr 25;38(17):4093-4103. doi: 10.1523/JNEUROSCI.2663-16.2018. Epub 2018 Mar 19. J Neurosci. 2018. PMID: 29555853 Free PMC article.
-
Impact of nuclear factor-κB on restoration of neuron growth and differentiation in hippocampus of degenerative brain.J Neurosci Res. 2015 Oct;93(10):1471-5. doi: 10.1002/jnr.23547. Epub 2015 Jan 13. J Neurosci Res. 2015. PMID: 25586448 Review.
-
TNF-α/NF-κB signaling in the CNS: possible connection to EPHB2.J Neuroimmune Pharmacol. 2014 Mar;9(2):133-41. doi: 10.1007/s11481-013-9517-x. Epub 2013 Nov 27. J Neuroimmune Pharmacol. 2014. PMID: 24277482 Free PMC article. Review.
-
IκB kinase/nuclear factor κB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling.J Neurosci. 2012 Apr 18;32(16):5688-703. doi: 10.1523/JNEUROSCI.0111-12.2012. J Neurosci. 2012. PMID: 22514330 Free PMC article.
Cited by
-
Heat Shock Protein 70 in Penile Neurovascular Regeneration Requires Cystathionine Gamma-Lyase.World J Mens Health. 2022 Oct;40(4):580-599. doi: 10.5534/wjmh.210249. Epub 2022 May 19. World J Mens Health. 2022. PMID: 36047068 Free PMC article.
-
Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth.Nat Neurosci. 2012 Jul 8;15(8):1134-43. doi: 10.1038/nn.3160. Nat Neurosci. 2012. PMID: 22772332
-
Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges.Immun Ageing. 2016 Apr 14;13:16. doi: 10.1186/s12979-016-0070-3. eCollection 2016. Immun Ageing. 2016. PMID: 27081392 Free PMC article. Review.
-
POTEE drives colorectal cancer development via regulating SPHK1/p65 signaling.Cell Death Dis. 2019 Nov 13;10(11):863. doi: 10.1038/s41419-019-2046-7. Cell Death Dis. 2019. PMID: 31723122 Free PMC article.
-
L-Lactate Regulates the Expression of Synaptic Plasticity and Neuroprotection Genes in Cortical Neurons: A Transcriptome Analysis.Front Mol Neurosci. 2018 Oct 10;11:375. doi: 10.3389/fnmol.2018.00375. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30364173 Free PMC article.
References
-
- Vallabhapurapu S., Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Ann. Rev. Immunol. 2009;27:693–733. - PubMed
-
- Memet S. NF-kappaB functions in the nervous system: from development to disease. Biochem. Pharmacol. 2006;72:1180–1195. - PubMed
-
- Romano A. Evolutionarily-conserved role of the NF-kappaB transcription factor in neural plasticity and memory. Eur. J. Neurosci. 2006;24:1507–1516. - PubMed
-
- Simpson C.S., Morris B.J. Regulation of neuronal cell adhesion molecule expression by NF-kappa B. J. Biol. Chem. 2000;275:16879–16884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

