Slit-2 facilitates interaction of P-cadherin with Robo-3 and inhibits cell migration in an oral squamous cell carcinoma cell line
- PMID: 21459757
- PMCID: PMC3314282
- DOI: 10.1093/carcin/bgr059
Slit-2 facilitates interaction of P-cadherin with Robo-3 and inhibits cell migration in an oral squamous cell carcinoma cell line
Abstract
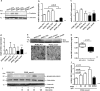
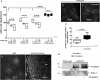
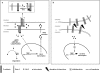
Slits are a group of secreted glycoproteins that act as molecular guidance cues in cellular migration. Recently, several studies demonstrated that Slit-2 can operate as candidate tumour suppressor protein in various tissues. In this study, we show Slit-2 expression in basal cell layers of normal oral mucosa colocalized with P-cadherin expression. In contrast, there is a loss of Slit-2 and P-cadherin expression in mucosa of oral squamous cell carcinoma (OSCC). Our in vitro investigations reveal a correlation of P-cadherin and Slit-2 expression: OSCC cells with induced P-cadherin expression (PCI52_PC) display an increased Slit-2 expression. However, abrogating P-cadherin function with a function-blocking antibody decreases Slit-2 secretion confirming a direct link between P-cadherin and Slit-2. Moreover, experiments with OSCC cells show that Slit-2 interferes with a Wnt related signalling pathway, which in turn affects Slit-2 expression in a feedback loop. Functionally, transwell migration assays demonstrate a Slit-2 dose-dependent decrease of PCI52_PC cell migration. However, there is no influence on migration in mock control cells. Responsible for this migration block might be an interaction of P-cadherin with Roundabout (Robo)-3, a high affinity receptor of Slit-2. Indeed, proximity ligation assays exhibit P-cadherin/Robo-3 interactions on PCI52_PC cells. Additionally, we detect a modulation of this interaction by addition of recombinant Slit-2. Down-regulation of Robo-3 expression via small interfering RNA neutralizes Slit-2 induced migration block in PCI52_PC cells. In summary, our experiments show antitumorigenic effects of Slit-2 on P-cadherin expressing OSCC cells supposedly via modulation of Robo-3 interaction.
Figures


References
-
- Bray F, et al. Estimates of cancer incidence and mortality in Europe in 1995. Eur. J. Cancer. 2002;38:99–166. - PubMed
-
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 1990;59:237–252. - PubMed
-
- Wheelock MJ, et al. Cadherin-mediated cellular signaling. Curr. Opin. Cell Biol. 2003;15:509–514. - PubMed
-
- Shimoyama Y, et al. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–2133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

