Saccade-related modulations of neuronal excitability support synchrony of visually elicited spikes
- PMID: 21459839
- PMCID: PMC3183421
- DOI: 10.1093/cercor/bhr020
Saccade-related modulations of neuronal excitability support synchrony of visually elicited spikes
Abstract
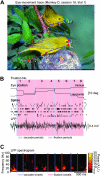
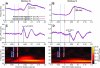
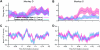
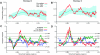
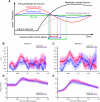
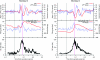
During natural vision, primates perform frequent saccadic eye movements, allowing only a narrow time window for processing the visual information at each location. Individual neurons may contribute only with a few spikes to the visual processing during each fixation, suggesting precise spike timing as a relevant mechanism for information processing. We recently found in V1 of monkeys freely viewing natural images, that fixation-related spike synchronization occurs at the early phase of the rate response after fixation-onset, suggesting a specific role of the first response spikes in V1. Here, we show that there are strong local field potential (LFP) modulations locked to the onset of saccades, which continue into the successive fixation periods. Visually induced spikes, in particular the first spikes after the onset of a fixation, are locked to a specific epoch of the LFP modulation. We suggest that the modulation of neural excitability, which is reflected by the saccade-related LFP changes, serves as a corollary signal enabling precise timing of spikes in V1 and thereby providing a mechanism for spike synchronization.
Figures




References
-
- Abeles M. Role of the cortical neuron: integrator or coincidence detector? Isr J Med Sci. 1982;18:83–92. - PubMed
-
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. - PubMed
-
- Bartlett JR, Doty RW, Lee BB, Sr, Sakakura H. Influence of saccadic eye movements on geniculostriate excitability in normal monkeys. Exp Brain Res. 1976;25:487–509. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

