Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells
- PMID: 21460038
- PMCID: PMC3070936
- DOI: 10.1101/gad.2005511
Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells
Abstract
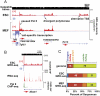
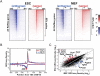
Transitions between pluripotent stem cells and differentiated cells are executed by key transcription regulators. Comparative measurements of RNA polymerase distribution over the genome's primary transcription units in different cell states can identify the genes and steps in the transcription cycle that are regulated during such transitions. To identify the complete transcriptional profiles of RNA polymerases with high sensitivity and resolution, as well as the critical regulated steps upon which regulatory factors act, we used genome-wide nuclear run-on (GRO-seq) to map the density and orientation of transcriptionally engaged RNA polymerases in mouse embryonic stem cells (ESCs) and mouse embryonic fibroblasts (MEFs). In both cell types, progression of a promoter-proximal, paused RNA polymerase II (Pol II) into productive elongation is a rate-limiting step in transcription of ∼40% of mRNA-encoding genes. Importantly, quantitative comparisons between cell types reveal that transcription is controlled frequently at paused Pol II's entry into elongation. Furthermore, "bivalent" ESC genes (exhibiting both active and repressive histone modifications) bound by Polycomb group complexes PRC1 (Polycomb-repressive complex 1) and PRC2 show dramatically reduced levels of paused Pol II at promoters relative to an average gene. In contrast, bivalent promoters bound by only PRC2 allow Pol II pausing, but it is confined to extremely 5' proximal regions. Altogether, these findings identify rate-limiting targets for transcription regulation during cell differentiation.
Figures





References
-
- Adelman K, Marr MT, Werner J, Saunders A, Ni Z, Andrulis ED, Lis JT 2005. Efficient release from promoter-proximal stall sites requires transcript cleavage factor TFIIS. Mol Cell 17: 103–112 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
