Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition
- PMID: 21460039
- PMCID: PMC3070937
- DOI: 10.1101/gad.2028911
Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition
Abstract
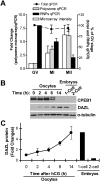
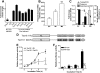
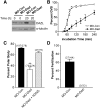
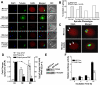
Oocyte maturation, fertilization, and early embryonic development occur in the absence of gene transcription. Therefore, it is critical to understand at a global level the post-transcriptional events that are driving these transitions. Here we used a systems approach by combining polysome mRNA profiling and bioinformatics to identify RNA-binding motifs in mRNAs that either enter or exit the polysome pool during mouse oocyte maturation. Association of mRNA with the polysomes correlates with active translation. Using this strategy, we identified highly specific patterns of mRNA recruitment to the polysomes that are synchronized with the cell cycle. A large number of the mRNAs recovered with translating ribosomes contain motifs for the RNA-binding proteins DAZL (deleted in azoospermia-like) and CPEB (cytoplasmic polyadenylation element-binding protein). Although a Dazl role in early germ cell development is well established, no function has been described during oocyte-to-embryo transition. We demonstrate that CPEB1 regulates Dazl post-transcriptionally, and that DAZL is essential for meiotic maturation and embryonic cleavage. In the absence of DAZL synthesis, the meiotic spindle fails to form due to disorganization of meiotic microtubules. Therefore, Cpeb1 and Dazl function in a progressive, self-reinforcing pathway to promote oocyte maturation and early embryonic development.
Figures


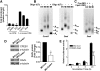
References
-
- Bayliss R, Sardon T, Vernos I, Conti E 2003. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell 12: 851–862 - PubMed
-
- Belloc E, Mendez R 2008. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature 452: 1017–1021 - PubMed
-
- Belloc E, Pique M, Mendez R 2008. Sequential waves of polyadenylation and deadenylation define a translation circuit that drives meiotic progression. Biochem Soc Trans 36: 665–670 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
