A new role for the dynamin GTPase in the regulation of fusion pore expansion
- PMID: 21460182
- PMCID: PMC3103406
- DOI: 10.1091/mbc.E11-02-0101
A new role for the dynamin GTPase in the regulation of fusion pore expansion
Abstract
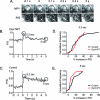
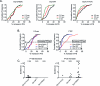
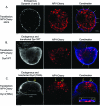
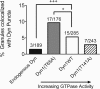
Dynamin is a master regulator of membrane fission in endocytosis. However, a function for dynamin immediately upon fusion has also been suspected from a variety of experiments that measured release of granule contents. The role of dynamin guanosine triphosphate hydrolase (GTPase) activity in controlling fusion pore expansion and postfusion granule membrane topology was investigated using polarization optics and total internal reflection fluorescence microscopy (pTIRFM) and amperometry. A dynamin-1 (Dyn1) mutant with increased GTPase activity resulted in transient deformations consistent with rapid fusion pore widening after exocytosis; a Dyn1 mutant with decreased activity slowed fusion pore widening by stabilizing postfusion granule membrane deformations. The experiments indicate that, in addition to its role in endocytosis, GTPase activity of dynamin regulates the rapidity of fusion pore expansion from tens of milliseconds to seconds after fusion. These findings expand the membrane-sculpting repertoire of dynamin to include the regulation of immediate postfusion events in exocytosis that control the rate of release of soluble granule contents.
Figures



References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-NS053978/NS/NINDS NIH HHS/United States
- F32GM086169/GM/NIGMS NIH HHS/United States
- R01 DK077050/DK/NIDDK NIH HHS/United States
- R37 MH061345/MH/NIMH NIH HHS/United States
- F32 GM086169/GM/NIGMS NIH HHS/United States
- MH61345/MH/NIMH NIH HHS/United States
- R56 DK077050/DK/NIDDK NIH HHS/United States
- T32 DA007268/DA/NIDA NIH HHS/United States
- R01 NS053978/NS/NINDS NIH HHS/United States
- R01 GM042455/GM/NIGMS NIH HHS/United States
- T32DA007268/DA/NIDA NIH HHS/United States
- R01 MH061345/MH/NIMH NIH HHS/United States
- R01 NS038129/NS/NINDS NIH HHS/United States
- R01-NS38129/NS/NINDS NIH HHS/United States
- GM42455/GM/NIGMS NIH HHS/United States
- DK077050/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

