Phosphorylation of STEF/Tiam2 by protein kinase A is critical for Rac1 activation and neurite outgrowth in dibutyryl cAMP-treated PC12D cells
- PMID: 21460187
- PMCID: PMC3093328
- DOI: 10.1091/mbc.E10-09-0783
Phosphorylation of STEF/Tiam2 by protein kinase A is critical for Rac1 activation and neurite outgrowth in dibutyryl cAMP-treated PC12D cells
Abstract
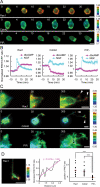
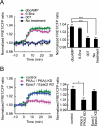
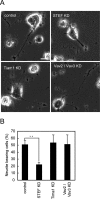
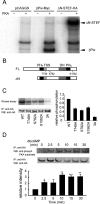
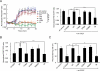
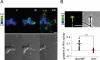
The second messenger cAMP plays a pivotal role in neurite/axon growth and guidance, but its downstream pathways leading to the regulation of Rho GTPases, centrally implicated in neuronal morphogenesis, remain elusive. We examined spatiotemporal changes in Rac1 and Cdc42 activity and phosphatidylinositol 3,4,5-triphosphate (PIP(3)) concentration in dibutyryl cAMP (dbcAMP)-treated PC12D cells using Förster resonance energy transfer-based biosensors. During a 30-min incubation with dbcAMP, Rac1 activity gradually increased throughout the cells and remained at its maximal level. There was no change in PIP(3) concentration. After a 5-h incubation with dbcAMP, Rac1 and Cdc42 were activated at the protruding tips of neurites without PIP(3) accumulation. dbcAMP-induced Rac1 activation was principally mediated by protein kinase A (PKA) and Sif- and Tiam1-like exchange factor (STEF)/Tiam2. STEF depletion drastically reduced dbcAMP-induced neurite outgrowth. PKA phosphorylates STEF at three residues (Thr-749, Ser-782, Ser-1562); Thr-749 phosphorylation was critical for dbcAMP-induced Rac1 activation and neurite extension. During dbcAMP-induced neurite outgrowth, PKA activation at the plasma membrane became localized to neurite tips; this localization may contribute to local Rac1 activation at the same neurite tips. Considering the critical role of Rac1 in neuronal morphogenesis, the PKA-STEF-Rac1 pathway may play a crucial role in cytoskeletal regulation during neurite/axon outgrowth and guidance, which depend on cAMP signals.
Figures
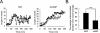
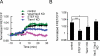
Similar articles
-
Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells.Mol Biol Cell. 2005 May;16(5):2207-17. doi: 10.1091/mbc.e04-10-0904. Epub 2005 Feb 23. Mol Biol Cell. 2005. PMID: 15728722 Free PMC article.
-
Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth.J Biol Chem. 2002 Jan 25;277(4):2860-8. doi: 10.1074/jbc.M106186200. Epub 2001 Nov 13. J Biol Chem. 2002. PMID: 11707441
-
NGF-dependent formation of ruffles in PC12D cells required a different pathway from that for neurite outgrowth.Neurochem Int. 2007 Jul-Sep;51(2-4):216-26. doi: 10.1016/j.neuint.2007.04.032. Epub 2007 May 17. Neurochem Int. 2007. PMID: 17561310
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
Mechanisms and consequences of dysregulation of the Tiam family of Rac activators in disease.Biochem Soc Trans. 2020 Dec 18;48(6):2703-2719. doi: 10.1042/BST20200481. Biochem Soc Trans. 2020. PMID: 33200195 Review.
Cited by
-
1-42 β-amyloid peptide requires PDK1/nPKC/Rac 1 pathway to induce neuronal death.Transl Psychiatry. 2013 Jan 22;3(1):e219. doi: 10.1038/tp.2012.147. Transl Psychiatry. 2013. PMID: 23340502 Free PMC article.
-
Genetically encoded fluorescent biosensors for live-cell visualization of protein phosphorylation.Chem Biol. 2014 Feb 20;21(2):186-97. doi: 10.1016/j.chembiol.2013.12.012. Epub 2014 Jan 30. Chem Biol. 2014. PMID: 24485761 Free PMC article. Review.
-
DDX3 Modulates Neurite Development via Translationally Activating an RNA Regulon Involved in Rac1 Activation.J Neurosci. 2016 Sep 21;36(38):9792-804. doi: 10.1523/JNEUROSCI.4603-15.2016. J Neurosci. 2016. PMID: 27656019 Free PMC article.
-
Regulating Rac in the nervous system: molecular function and disease implication of Rac GEFs and GAPs.Biomed Res Int. 2015;2015:632450. doi: 10.1155/2015/632450. Epub 2015 Mar 24. Biomed Res Int. 2015. PMID: 25879033 Free PMC article. Review.
-
Loss-of-function variants in TIAM1 are associated with developmental delay, intellectual disability, and seizures.Am J Hum Genet. 2022 Apr 7;109(4):571-586. doi: 10.1016/j.ajhg.2022.01.020. Epub 2022 Mar 2. Am J Hum Genet. 2022. PMID: 35240055 Free PMC article.
References
-
- Aglah C, Gordon T, Posse de Chaves EI. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacol. 2008;55:8–17. - PubMed
-
- Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun. 2006;348:716–721. - PubMed
-
- Aoki K, Nakamura T, Matsuda M. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J Biol Chem. 2004;279:713–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

