Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase
- PMID: 21460217
- PMCID: PMC3099729
- DOI: 10.1074/jbc.M111.223677
Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase
Abstract
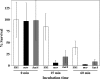
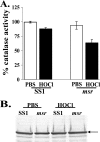
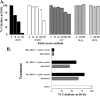
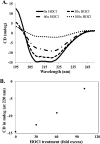
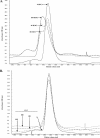
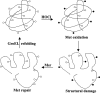
Hypochlorous acid (HOCl) produced via the enzyme myeloperoxidase is a major antibacterial oxidant produced by neutrophils, and Met residues are considered primary amino acid targets of HOCl damage via conversion to Met sulfoxide. Met sulfoxide can be repaired back to Met by methionine sulfoxide reductase (Msr). Catalase is an important antioxidant enzyme; we show it constitutes 4-5% of the total Helicobacter pylori protein levels. msr and katA strains were about 14- and 4-fold, respectively, more susceptible than the parent to killing by the neutrophil cell line HL-60 cells. Catalase activity of an msr strain was much more reduced by HOCl exposure than for the parental strain. Treatment of pure catalase with HOCl caused oxidation of specific MS-identified Met residues, as well as structural changes and activity loss depending on the oxidant dose. Treatment of catalase with HOCl at a level to limit structural perturbation (at a catalase/HOCl molar ratio of 1:60) resulted in oxidation of six identified Met residues. Msr repaired these residues in an in vitro reconstituted system, but no enzyme activity could be recovered. However, addition of GroEL to the Msr repair mixture significantly enhanced catalase activity recovery. Neutrophils produce large amounts of HOCl at inflammation sites, and bacterial catalase may be a prime target of the host inflammatory response; at high concentrations of HOCl (1:100), we observed loss of catalase secondary structure, oligomerization, and carbonylation. The same HOCl-sensitive Met residue oxidation targets in catalase were detected using chloramine-T as a milder oxidant.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

