Multiple mechanisms collaborate to repress nanos translation in the Drosophila ovary and embryo
- PMID: 21460235
- PMCID: PMC3078745
- DOI: 10.1261/rna.2478611
Multiple mechanisms collaborate to repress nanos translation in the Drosophila ovary and embryo
Abstract
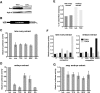
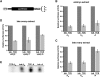
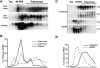
Translational control of gene expression is essential for development in organisms that rely on maternal mRNAs. In Drosophila, translation of maternal nanos (nos) mRNA must be restricted to the posterior of the early embryo for proper patterning of the anterior-posterior axis. Spatial control of nos translation is coordinated through the localization of a small subset of nos mRNA to the posterior pole late in oogenesis, activation of this localized mRNA, and repression of the remaining unlocalized nos mRNA throughout the bulk cytoplasm. Translational repression is mediated by the interaction of a cis-acting element in the nos 3' untranslated region with two proteins, Glorund (Glo) and Smaug (Smg), that function in the oocyte and embryo, respectively. The mechanism of Glo-dependent repression is unknown. Previous work suggests that Smg represses translation initiation but this model is not easily reconciled with evidence for polysome association of repressed nos mRNA. Using an in vitro translation system, we have decoupled translational repression of nos imposed during oogenesis from repression during embryogenesis. Our results suggest that both Glo and Smg regulate translation initiation, but by different mechanisms. Furthermore, we show that, during late oogenesis, nos translation is also repressed post-initiation and provide evidence that Glo mediates this event. This post-initiation block is maintained into embryogenesis during the transition to Smg-dependent regulation. We propose that the use of multiple modes of repression ensures inactivation of nos RNA that is translated at earlier stages of oogenesis and maintenance of this inactivate state throughout late oogenesis into embryogenesis.
Figures



References
-
- Bergsten SE, Gavis ER 1999. Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development 126: 659–669 - PubMed
-
- Chekulaeva M, Hentze MW, Ephrussi A 2006. Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell 124: 521–533 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
