Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies
- PMID: 21460314
- PMCID: PMC3070035
- DOI: 10.1093/cid/cir121
Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies
Abstract
Background: Lack of life-long immunity against influenza viruses represents a major global health care problem with profound medical and economic consequences. A greater understanding of the broad-spectrum "heterosubtypic" neutralizing human antibody (BnAb) response to influenza should bring us closer toward a universal influenza vaccine.
Methods: Serum samples obtained from 77 volunteers in an H5N1 vaccine study were analyzed for cross-reactive antibodies (Abs) against both subtype hemagglutinins (HAs) and a highly conserved pocket on the HA stem of Group 1 viruses. Cross-reactive Abs in commercial intravenous immunoglobulin were affinity purified using H5-coupled beads followed by step-wise monoclonal antibody competition or acid elution. Enzyme-linked immunosorbent assays were used to quantify cross-binding, and neutralization activity was determined with HA-pseudotyped viruses.
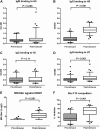
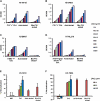
Results: Prevaccination serum samples have detectable levels of heterosubtypic HA binding activity to both Group 1 and 2 influenza A viruses, including subtypes H5 and H7, respectively, to which study subjects had not been vaccinated. Two different populations of Broadly neutralizing Abs (BnAbs) were purified from intravenous immunoglobulin by H5 beads: ~0.01% of total immunoglobulin G can bind to HAs from both Group 1 and 2 and neutralize H1N1 and H5N1 viruses; ~0.001% is F10-like Abs directed against the HA stem pocket on Group 1 viruses.
Conclusion: These data--to our knowledge, for the first time--quantitatively show the presence, albeit at low levels, of two populations of heterosubtypic BnAbs against influenza A in human serum. These observations warrant further investigation to determine their origin, host polymorphism(s) that may affect their expression levels and how to boost these BnAb responses by vaccination to reach sustainable protective levels.
Figures
Comment in
-
Prospecting the influenza hemagglutinin to develop universal vaccines.Clin Infect Dis. 2011 Apr 15;52(8):1010-2. doi: 10.1093/cid/cir129. Clin Infect Dis. 2011. PMID: 21460315 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

