Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants
- PMID: 21460326
- PMCID: PMC3070029
- DOI: 10.1093/cid/cir008
Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants
Abstract
Background: The World Health Organization currently recommends initiation of highly active antiretroviral therapy (HAART) for human immunodeficiency virus (HIV)-infected lactating women with CD4+ cell counts <350 cells/μL or stage 3 or 4 disease. We analyzed antiretroviral drug resistance in HIV-infected infants in the Post Exposure Prophylaxis of Infants trial whose mothers initiated HAART postpartum (with a regimen of nevirapine [NVP], stavudine, and lamivudine). Infants in the trial received single-dose NVP and a week of zidovudine (ZDV) at birth; some infants also received extended daily NVP prophylaxis, with or without extended ZDV prophylaxis.
Methods: We analyzed drug resistance in plasma samples collected from all HIV-infected infants whose mothers started HAART in the first postpartum year. Resistance testing was performed using the first plasma sample collected within 6 months after maternal HAART initiation. Categorical variables were compared by exact or trend tests; continuous variables were compared using rank-sum tests.
Results: Multiclass resistance (MCR) was detected in HIV from 11 (29.7%) of 37 infants. Infants were more likely to develop MCR infection if their mothers initiated HAART earlier in the postpartum period (by 14 weeks vs after 14 weeks and up to 6 months vs after 6 months, P = .0009), or if the mother was exclusively breastfeeding at the time of HAART initiation (exclusive breastfeeding vs mixed feeding vs no breastfeeding, P = .003).
Conclusions: Postpartum maternal HAART initiation was associated with acquisition of MCR in HIV-infected breastfeeding infants. The risk was higher among infants whose mothers initiated HAART closer to the time of delivery or were still exclusively breastfeeding when they first reported HAART use.
Figures

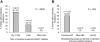
References
-
- Mofenson LM. Protecting the next generation–eliminating perinatal HIV-1 infection. N Engl J Med. 2010;362:2316–2318. - PubMed
-
- World Health Organization (WHO) HIV and infant feeding. Rapid advice. 2009. Available at: http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf. Accessed 5 March 2010.
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization. AIDS Epidemic Update. Geneva, Switzerland: UNAIDS; 2009. Available at: http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. Accessed 6 June 2010.
-
- Kuhn L, Reitz C, Abrams EJ. Breastfeeding and AIDS in the developing world. Curr Opin Pediatr. 2009;21:83–93. - PubMed
-
- Kilewo C, Karlsson K, Massawe A, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. J Acquir Immune Defic Syndr. 2008;48:315–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

