Neuronal activity is required for the development of specific cortical interneuron subtypes
- PMID: 21460837
- PMCID: PMC3641515
- DOI: 10.1038/nature09865
Neuronal activity is required for the development of specific cortical interneuron subtypes
Abstract
Electrical activity has been shown to regulate development in a variety of species and in various structures, including the retina, spinal cord and cortex. Within the mammalian cortex specifically, the development of dendrites and commissural axons in pyramidal cells is activity-dependent. However, little is known about the developmental role of activity in the other major cortical population of neurons, the GABA-producing interneurons. These neurons are morphologically and functionally heterogeneous and efforts over the past decade have focused on determining the mechanisms that contribute to this diversity. It was recently discovered that 30% of all cortical interneurons arise from a relatively novel source within the ventral telencephalon, the caudal ganglionic eminence (CGE). Owing to their late birth date, these interneurons populate the cortex only after the majority of other interneurons and pyramidal cells are already in place and have started to functionally integrate. Here we demonstrate in mice that for CGE-derived reelin (Re)-positive and calretinin (Cr)-positive (but not vasoactive intestinal peptide (VIP)-positive) interneurons, activity is essential before postnatal day 3 for correct migration, and that after postnatal day 3, glutamate-mediated activity controls the development of their axons and dendrites. Furthermore, we show that the engulfment and cell motility 1 gene (Elmo1), a target of the transcription factor distal-less homeobox 1 (Dlx1), is selectively expressed in Re(+) and Cr(+) interneurons and is both necessary and sufficient for activity-dependent interneuron migration. Our findings reveal a selective requirement for activity in shaping the cortical integration of specific neuronal subtypes.
Figures

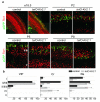


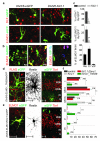
Comment in
-
Cortical development: Activity makes interneurons shape up.Nat Rev Neurosci. 2011 Jun;12(6):308. doi: 10.1038/nrn3046. Epub 2011 May 18. Nat Rev Neurosci. 2011. PMID: 21587287 No abstract available.
Similar articles
-
Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons.J Neurosci. 2015 Sep 16;35(37):12869-89. doi: 10.1523/JNEUROSCI.1164-15.2015. J Neurosci. 2015. PMID: 26377473 Free PMC article.
-
Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons.J Neurosci. 2010 Feb 3;30(5):1582-94. doi: 10.1523/JNEUROSCI.4515-09.2010. J Neurosci. 2010. PMID: 20130169 Free PMC article.
-
Cux-1 and Cux-2 control the development of Reelin expressing cortical interneurons.Dev Neurobiol. 2008 Jun;68(7):917-25. doi: 10.1002/dneu.20626. Dev Neurobiol. 2008. PMID: 18327765 Free PMC article.
-
Building a human cortex: the evolutionary differentiation of Cajal-Retzius cells and the cortical hem.J Anat. 2010 Oct;217(4):334-43. doi: 10.1111/j.1469-7580.2010.01266.x. J Anat. 2010. PMID: 20626498 Free PMC article. Review.
-
Cortical interneuron fate determination: diverse sources for distinct subtypes?Cereb Cortex. 2003 Jun;13(6):670-6. doi: 10.1093/cercor/13.6.670. Cereb Cortex. 2003. PMID: 12764043 Review.
Cited by
-
In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons.Nat Neurosci. 2013 Feb;16(2):193-200. doi: 10.1038/nn.3299. Epub 2013 Jan 6. Nat Neurosci. 2013. PMID: 23292682
-
Neural Signaling in Cancer.Annu Rev Neurosci. 2022 Jul 8;45:199-221. doi: 10.1146/annurev-neuro-111020-092702. Epub 2022 Mar 8. Annu Rev Neurosci. 2022. PMID: 35259916 Free PMC article. Review.
-
In vivo rapid gene delivery into postmitotic neocortical neurons using iontoporation.Nat Protoc. 2015 Jan;10(1):25-32. doi: 10.1038/nprot.2015.001. Epub 2014 Dec 4. Nat Protoc. 2015. PMID: 25474030
-
Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons.Dev Cell. 2014 Sep 29;30(6):645-59. doi: 10.1016/j.devcel.2014.07.001. Epub 2014 Sep 4. Dev Cell. 2014. PMID: 25199688 Free PMC article.
-
Developmental Sculpting of Intracortical Circuits by MHC Class I H2-Db and H2-Kb.Cereb Cortex. 2016 Apr;26(4):1453-1463. doi: 10.1093/cercor/bhu243. Epub 2014 Oct 14. Cereb Cortex. 2016. PMID: 25316337 Free PMC article.
References
-
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. - PubMed
-
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. - PubMed
-
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

