Structure of catalytically competent intein caught in a redox trap with functional and evolutionary implications
- PMID: 21460844
- PMCID: PMC3087850
- DOI: 10.1038/nsmb.2041
Structure of catalytically competent intein caught in a redox trap with functional and evolutionary implications
Abstract
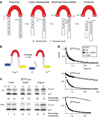
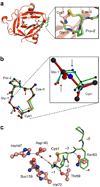
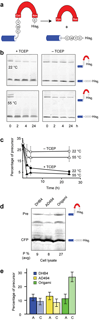
Here we describe self-splicing proteins, called inteins, that function as redox-responsive switches in bacteria. Redox regulation was achieved by engineering a disulfide bond between the intein's catalytic cysteine and a cysteine in the flanking 'extein' sequence. This interaction was validated by an X-ray structure, which includes a transient splice junction. A natural analog of the designed system was identified in Pyrococcus abyssi, suggesting an unprecedented form of adaptive, post-translational regulation.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

