The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury
- PMID: 21460863
- PMCID: PMC4002449
- DOI: 10.1038/cmi.2011.8
The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury
Abstract
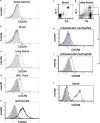
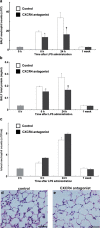
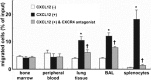
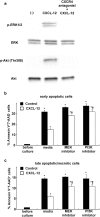
Inflammatory stimuli, such as a microbes or lipopolysaccharides, induce a rapid release of neutrophils from the bone marrow and promote neutrophil migration into inflamed sites to promote host defense. However, an excess accumulation and retention of neutrophils in inflamed tissue can cause severe tissue injuries in the later stages of inflammation. Recent studies have reported that both CXCL12 levels in injured lungs and its receptor, CXCR4, on accumulated neutrophils in injured lungs, increased; furthermore, these studies showed that the CXCL12/CXCR4 signaling pathway participated in neutrophil accumulation in the later stages of lipopolysaccharide (LPS)-induced lung injury. However, the mechanisms underlying this increase in surface CXCR4 expression in neutrophils remain unclear. In this study, we found that surface CXCR4 expression increased in extravascular, but not intravascular, neutrophils in the lungs of LPS-induced lung injury model mice. Furthermore, ex vivo studies revealed that CXCL12 acted not only as a chemoattractant, but also as a suppressor of cell death for the lung neutrophils expressing CXCR4. Sulfatide, one of the native ligands for L-selectin, induced the increase of surface CXCR4 expression on isolated circulating neutrophils, suggesting that the activation of L-selectin may be involved in the increase in surface CXCR4. Our findings show that surface CXCR4 levels on neutrophils increase after extravasation into injured lungs, possibly through the activation of L-selectin. The CXCL12/CXCR4 signaling pathway plays an important role in the modulation of neutrophil activity during acute lung injury, not only by promoting chemotaxis but also by suppressing cell death.
Figures

References
-
- Kubo H, Graham L, Doyle NA, Quinlan WM, Hogg JC, Doerschuk CM. Complement fragment-induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood. 1998;92:283–290. - PubMed
-
- Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447–S463. - PubMed
-
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. - PubMed
-
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

