Phasic motor activity of respiratory and non-respiratory muscles in REM sleep
- PMID: 21461320
- PMCID: PMC3065252
- DOI: 10.1093/sleep/34.4.425
Phasic motor activity of respiratory and non-respiratory muscles in REM sleep
Abstract
Objectives: In this study, we quantified the profiles of phasic activity in respiratory muscles (diaphragm, genioglossus and external intercostal) and non-respiratory muscles (neck and extensor digitorum) across REM sleep. We hypothesized that if there is a unique pontine structure that controls all REM sleep phasic events, the profiles of the phasic twitches of different muscle groups should be identical. Furthermore, we described how respiratory parameters (e.g., frequency, amplitude, and effort) vary across REM sleep to determine if phasic processes affect breathing.
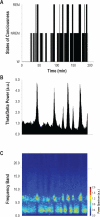
Methods: Electrodes were implanted in Wistar rats to record brain activity and muscle activity of neck, extensor digitorum, diaphragm, external intercostal, and genioglossal muscles. Ten rats were studied to obtain 313 REM periods over 73 recording days. Data were analyzed offline and REM sleep activity profiles were built for each muscle. In 6 animals, respiratory frequency, effort, amplitude, and inspiratory peak were also analyzed during 192 REM sleep periods.
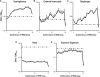
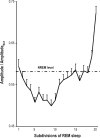
Results: Respiratory muscle phasic activity increased in the second part of the REM period. For example, genioglossal activity increased in the second part of the REM period by 63.8% compared to the average level during NREM sleep. This profile was consistent between animals and REM periods (η(2)=0.58). This increased activity seen in respiratory muscles appeared as irregular bursts and trains of activity that could affect rythmo-genesis. Indeed, the increased integrated activity seen in the second part of the REM period in the diaphragm was associated with an increase in the number (28.3%) and amplitude (30%) of breaths. Non-respiratory muscle phasic activity in REM sleep did not have a profile like the phasic activity of respiratory muscles. Time in REM sleep did not have an effect on nuchal activity (P=0.59).
Conclusion: We conclude that the concept of a common pontine center controlling all REM phasic events is not supported by our data. There is a drive in REM sleep that affects specifically respiratory muscles. The characteristic increase in respiratory frequency during REM sleep is induced by this drive.
Keywords: REM phasic event; diaphragm; external intercostal; genioglossus; rat.
Figures





References
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–4. - PubMed
-
- Bennett JR, Dunroy HM, Corfield DR. Respiratory muscle activity during REM sleep in patients with diaphragm paralysis. Neurology. 2004;62:134–7. - PubMed
-
- De Gennaro L, Ferrera M. Sleep deprivation and phasic activity of REM sleep: independence of middle-ear muscle activity from rapid eye movements. Sleep. 2000;23:81–5. - PubMed
-
- Douglas NJ. Sleep in patients with chronic obstructive pulmonary disease. Clin Chest Med. 1998;19:115–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

