A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial
- PMID: 21461326
- PMCID: PMC3065258
- DOI: 10.1093/sleep/34.4.479
A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial
Abstract
Study objectives: Investigate the efficacy of a novel nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA).
Design: A prospective, multicenter, sham-controlled, parallel-group, randomized, double-blind clinical trial.
Setting: 19 sites including both academic and private sleep disorder centers
Patients: Obstructive sleep apnea with a pre-study AHI ≥10/hour
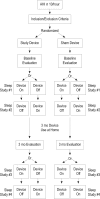
Interventions: Treatment with a nasal EPAP device (N=127) or similar appearing sham device (N=123) for 3 months. Polysomnography (PSG) was performed on 2 non-consecutive nights (random order: device-on, device-off) at week 1 and after 3 months of treatment. Analysis of an intention to treat group (ITT) (patients completing week 1 PSGs) (EPAP N=119, sham N=110) was performed.
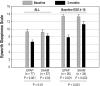
Measurements and results: At week 1, the median AHI value (device-on versus device-off) was significantly lower with EPAP (5.0 versus 13.8 events/h, P<0.0001) but not sham (11.6 versus 11.1 events/h, P=NS); the decrease in the AHI (median) was greater (-52.7% vs. -7.3%, P<0.0001) for the ITT group. At month 3, the percentage decrease in the AHI was 42.7% (EPAP) and 10.1% (sham), P<0.0001. Over 3 months of EPAP treatment the Epworth Sleepiness Scale decreased (9.9 ± 4.7 to 7.2 ± 4.2, P<0.0001), and the median percentage of reported nights used (entire night) was 88.2%.
Conclusions: The nasal EPAP device significantly reduced the AHI and improved subjective daytime sleepiness compared to the sham treatment in patients with mild to severe OSA with excellent adherence.
Registrations: ClinicalTrials.gov. Trial name: Randomized Study of Provent Versus Sham Device to Treat Obstructive Sleep Apnea (AERO). URL: http://www.clinicaltrials.gov/ct2/show/NCT00772044?term=Ventus&rank=1.
Registration number: NCT00772044.
Keywords: CPAP; Obstructive sleep apnea; expiratory positive airway pressure.
Figures
Similar articles
-
Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA).J Clin Sleep Med. 2011 Oct 15;7(5):449-53B. doi: 10.5664/JCSM.1304. J Clin Sleep Med. 2011. PMID: 22003339 Free PMC article. Clinical Trial.
-
Combination therapy with mandibular advancement and expiratory positive airway pressure valves reduces obstructive sleep apnea severity.Sleep. 2019 Aug 1;42(8):zsz119. doi: 10.1093/sleep/zsz119. Sleep. 2019. PMID: 31180512
-
A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence.J Clin Sleep Med. 2009 Dec 15;5(6):532-7. J Clin Sleep Med. 2009. PMID: 20465019 Free PMC article. Clinical Trial.
-
A review of EPAP nasal device therapy for obstructive sleep apnea syndrome.Sleep Breath. 2015 Sep;19(3):769-74. doi: 10.1007/s11325-014-1057-y. Epub 2014 Sep 23. Sleep Breath. 2015. PMID: 25245174 Review.
-
High- and low-intensity expiratory muscle strength training in patients with severe obstructive sleep apnea syndrome using non-invasive mechanical ventilation: A double-blinded, randomized controlled trial.Heart Lung. 2023 Sep-Oct;61:29-36. doi: 10.1016/j.hrtlng.2023.03.009. Epub 2023 Apr 21. Heart Lung. 2023. PMID: 37087896 Review.
Cited by
-
New and unconventional treatments for obstructive sleep apnea.Neurotherapeutics. 2012 Oct;9(4):702-9. doi: 10.1007/s13311-012-0146-5. Neurotherapeutics. 2012. PMID: 22987061 Free PMC article. Review.
-
Expiratory Positive Airway Pressure for Sleep Apnea after Stroke: A Randomized, Crossover Trial.J Clin Sleep Med. 2016 Sep 15;12(9):1233-8. doi: 10.5664/jcsm.6120. J Clin Sleep Med. 2016. PMID: 27306393 Free PMC article. Clinical Trial.
-
Alternative devices for obstructive sleep apnea.Neurol Clin Pract. 2013 Feb;3(1):67-70. doi: 10.1212/CPJ.0b013e318278be88. Neurol Clin Pract. 2013. PMID: 29406530 Free PMC article.
-
Innovative treatments for adults with obstructive sleep apnea.Nat Sci Sleep. 2014 Nov 18;6:137-47. doi: 10.2147/NSS.S46818. eCollection 2014. Nat Sci Sleep. 2014. PMID: 25429246 Free PMC article. Review.
-
Consensus & evidence-based INOSA Guidelines 2014 (first edition).Indian J Med Res. 2014 Sep;140(3):451-68. Indian J Med Res. 2014. PMID: 25366217 Free PMC article.
References
-
- Young T, Palta M, Dempsey J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. - PubMed
-
- Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. - PubMed
-
- Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomized prospective parallel trial. Lancet. 1999;353:2100–5. - PubMed
-
- Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

