Female reproductive hormones alter sleep architecture in ovariectomized rats
- PMID: 21461331
- PMCID: PMC3065263
- DOI: 10.1093/sleep/34.4.519
Female reproductive hormones alter sleep architecture in ovariectomized rats
Erratum in
- Sleep. 2011;34(7):976
Abstract
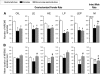
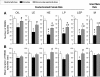
Study objectives: Treating ovariectomized rats with physiological levels of estradiol and/or progesterone affects aspects of both baseline (24 h) sleep and recovery (18 h) sleep after 6 h of sleep deprivation. We have extended the analysis of these effects by examining several additional parameters of sleep architecture using the same data set as in our previous study (Deurveilher et al. SLEEP 2009;32(7):865-877).
Design: Sleep in ovariectomized rats implanted with oil, 17 β-estradiol and/or progesterone capsules was recorded using EEG and EMG before, during, and after 6 h of sleep deprivation during the light phase of a 12/12 h light/dark cycle.
Measurements and results: During the baseline dark, but not light, phase, treatments with estradiol alone or combined with progesterone decreased the mean duration of non-rapid eye movement sleep (NREMS) episodes and the number of REMS episodes, while also increasing brief awakenings, consistent with the previously reported lower baseline NREMS and REMS amounts. Following sleep deprivation, the hormonal treatments caused a larger percentage increase from baseline in the mean durations of NREMS and REMS episodes, and a larger percentage decrease in brief awakenings, consistent with the previously reported larger increase in recovery REMS amount. There were no hormonal effects on NREMS and REMS EEG power values, other than on recovery NREMS delta power, as previously reported.
Conclusions: Physiological levels of estradiol and/or progesterone in female rats modulate sleep architecture differently at baseline and after acute sleep loss, fragmenting baseline sleep while consolidating recovery sleep. These hormones also play a role in the diurnal pattern of NREMS maintenance.
Keywords: Ovariectomy; estradiol; progesterone; sleep architecture; sleep deprivation; sleep homeostasis.
Figures
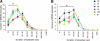
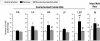
Similar articles
-
Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats.Sleep. 2009 Jul;32(7):865-77. Sleep. 2009. PMID: 19639749 Free PMC article.
-
Ovarian hormones promote recovery from sleep deprivation by increasing sleep intensity in middle-aged ovariectomized rats.Horm Behav. 2013 Apr;63(4):566-76. doi: 10.1016/j.yhbeh.2013.02.011. Epub 2013 Feb 27. Horm Behav. 2013. PMID: 23454003
-
Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats.Physiol Behav. 2011 Oct 24;104(5):962-71. doi: 10.1016/j.physbeh.2011.06.016. Epub 2011 Jun 23. Physiol Behav. 2011. PMID: 21722658 Free PMC article.
-
Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system.J Sleep Res. 2004 Sep;13(3):179-208. doi: 10.1111/j.1365-2869.2004.00412.x. J Sleep Res. 2004. PMID: 15339255 Review.
-
Delta wave power: an independent sleep phenotype or epiphenomenon?J Clin Sleep Med. 2011 Oct 15;7(5 Suppl):S16-8. doi: 10.5664/JCSM.1346. J Clin Sleep Med. 2011. PMID: 22003323 Free PMC article. Review.
Cited by
-
The importance of including both sexes in preclinical sleep studies and analyses.Sci Rep. 2024 Oct 15;14(1):23622. doi: 10.1038/s41598-024-70996-1. Sci Rep. 2024. PMID: 39406742 Free PMC article.
-
A review of the current state of knowledge on sex differences in sleep and circadian phenotypes in rodents.Neurobiol Sleep Circadian Rhythms. 2021 Jun 13;11:100068. doi: 10.1016/j.nbscr.2021.100068. eCollection 2021 Nov. Neurobiol Sleep Circadian Rhythms. 2021. PMID: 34195482 Free PMC article. Review.
-
Sleep in Women Across the Life Span.Chest. 2018 Jul;154(1):196-206. doi: 10.1016/j.chest.2018.04.005. Epub 2018 Apr 19. Chest. 2018. PMID: 29679598 Free PMC article. Review.
-
Impacts of traumatic brain injury severity and sex on sleep architecture, duration, and fragmentation.Neurobiol Sleep Circadian Rhythms. 2025 May 15;18:100127. doi: 10.1016/j.nbscr.2025.100127. eCollection 2025 May. Neurobiol Sleep Circadian Rhythms. 2025. PMID: 40495996 Free PMC article.
-
Influence of sex hormone use on sleep architecture in a transgender cohort.Sleep. 2023 Nov 8;46(11):zsad249. doi: 10.1093/sleep/zsad249. Sleep. 2023. PMID: 37715990 Free PMC article.
References
-
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. - PubMed
-
- Farage MA, Osborn TW, MacLean AB. Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Arch Gynecol Obstet. 2008;278:299–307. - PubMed
-
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci. 2005;1052:3–10. - PubMed
-
- Dzaja A, Arber S, Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

