Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein
- PMID: 21461375
- PMCID: PMC3065234
- DOI: 10.1155/2011/617892
Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein
Abstract
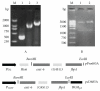
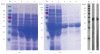
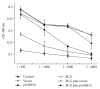
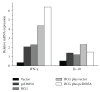
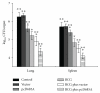
Heterologous prime-boost regimens utilizing BCG as a prime vaccine probably represent the best hope for the development of novel tuberculosis (TB) vaccines. In this study, we examined the immunogenicity and protective efficacy of DNA vaccine (pcD685A) expressing the fusion protein of Ag85A and ESAT-6 (r685A) and its booster effects in BCG-immunized mice. The recombinant r685A fusion protein stimulated higher level of antigen-specific IFN-γ release in tuberculin skin test- (TST-) positive healthy household contacts of active pulmonary TB patients than that in TST-negative population. Vaccination of C57BL/6 mice with pcD685A resulted in significant protection against challenge with virulent Mycobacterium tuberculosis H37Rv when compared with the control group. Most importantly, pcD685A could act as a BCG booster and amplify Th1-type cell-mediated immunity in the lung of BCG-vaccinated mice as shown the increased expression of IFN-γ. The most significant reduction in bacterial load of both spleen and lung was obtained in mice vaccinated with BCG prime and pcD685A DNA booster when compared with BCG or pcD685A alone. Thus, our study indicates that pcD685A may be an efficient booster vaccine against TB with a strong ability to enhance prior BCG immunity.
Figures



Similar articles
-
Comparison of BCG prime-DNA booster and rBCG regimens for protection against tuberculosis.Hum Vaccin Immunother. 2014;10(2):391-8. doi: 10.4161/hv.26969. Epub 2013 Nov 5. Hum Vaccin Immunother. 2014. PMID: 24192709 Free PMC article.
-
Protection against Mycobacterium tuberculosis challenge in mice by DNA vaccine Ag85A-ESAT-6-IL-21 priming and BCG boosting.Int J Immunogenet. 2012 Apr;39(2):183-90. doi: 10.1111/j.1744-313X.2011.01066.x. Epub 2011 Dec 8. Int J Immunogenet. 2012. PMID: 22152009
-
A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice.Med Microbiol Immunol. 2011 Aug;200(3):165-75. doi: 10.1007/s00430-011-0188-z. Epub 2011 Feb 22. Med Microbiol Immunol. 2011. PMID: 21340709
-
Vaccination against tuberculosis: how can we better BCG?Microb Pathog. 2013 May;58:2-16. doi: 10.1016/j.micpath.2012.12.002. Epub 2012 Dec 17. Microb Pathog. 2013. PMID: 23257069 Review.
-
Preclinical evidence for implementing a prime-boost vaccine strategy for tuberculosis.Vaccine. 2012 Apr 16;30(18):2811-23. doi: 10.1016/j.vaccine.2012.02.036. Epub 2012 Mar 3. Vaccine. 2012. PMID: 22387630 Free PMC article. Review.
Cited by
-
Protection against SHIV-KB9 infection by combining rDNA and rFPV vaccines based on HIV multiepitope and p24 protein in Chinese rhesus macaques.Clin Dev Immunol. 2012;2012:958404. doi: 10.1155/2012/958404. Epub 2012 Feb 26. Clin Dev Immunol. 2012. PMID: 22474488 Free PMC article.
-
A Mycobacterium bovis BCG-naked DNA prime-boost vaccination strategy induced CD4⁺ and CD8⁺ T-cell response against Mycobacterium tuberculosis immunogens.J Immunol Res. 2014;2014:395626. doi: 10.1155/2014/395626. Epub 2014 Mar 11. J Immunol Res. 2014. PMID: 24741595 Free PMC article.
-
Protection against Mycobacterium tuberculosis infection offered by a new multistage subunit vaccine correlates with increased number of IFN-γ+ IL-2+ CD4+ and IFN-γ+ CD8+ T cells.PLoS One. 2015 Mar 30;10(3):e0122560. doi: 10.1371/journal.pone.0122560. eCollection 2015. PLoS One. 2015. PMID: 25822536 Free PMC article.
-
Serodiagnosis efficacy and immunogenicity of the fusion protein of Mycobacterium tuberculosis composed of the 10-kilodalton culture filtrate protein, ESAT-6, and the extracellular domain fragment of PPE68.Clin Vaccine Immunol. 2012 Apr;19(4):536-44. doi: 10.1128/CVI.05708-11. Epub 2012 Feb 22. Clin Vaccine Immunol. 2012. PMID: 22357648 Free PMC article.
-
A Toxoplasma gondii vaccine encoding multistage antigens in conjunction with ubiquitin confers protective immunity to BALB/c mice against parasite infection.Parasit Vectors. 2015 Sep 30;8:498. doi: 10.1186/s13071-015-1108-7. Parasit Vectors. 2015. PMID: 26420606 Free PMC article.
References
-
- WHO. WHO Report. Geneva, Switzerland: 2009. Global tuberculosis control: a short update to the 2009 report.
-
- Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–1580. - PubMed
-
- BCG vaccine. WHO position paper. The Weekly Epidemiological Record. 2004;79:27–38. - PubMed
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–1180. - PubMed
-
- Fine PEM, Carneiro IAM, Milstien JB, Clements CJ. Issues Relating to the Use of BCG in Immunization Programmes. WHO, Geneva, Switzerland, 1999.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
