A Decade of Experience Using mTor Inhibitors in Liver Transplantation
- PMID: 21461386
- PMCID: PMC3064995
- DOI: 10.1155/2011/913094
A Decade of Experience Using mTor Inhibitors in Liver Transplantation
Abstract
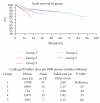
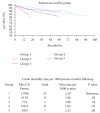
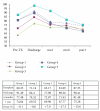
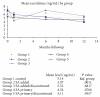
Some studies suggest that Sirolimus (SRL) is associated with an increased risk of death in liver transplant recipients compared to treatment with calcineurin inhibitors (CNIs). We compared patients who received SRL or CNI in the first year after liver transplant. Our database included 688 patients who received a liver transplant. The patients were divided into groups. (1) CNI + MPS (mycophenolate sodium) at time of discharge. (2) CNI + MPS at time of discharge; SRL was added within the first 6 months and continued through the first year. (3) CNI + MPS at time of discharge; SRL was added within the first 6 months and discontinued before the first year. (4) SRL as primary immunosuppression. (5) SRL as primary immunosuppression and discontinued before the first year. We used mortality and graft loss as the primary measures of outcome. We also quantified renal function using the change in glomerular filtration rate (GFR), the presence of biopsy proven acute cellular reject (ACR), and steroid-resistant rejection (SRR). There were no significant differences in mortality or graft loss. There was no difference in patient or graft survival. Patients that received SRL as primary immunosuppression had 50% less rejection compared to controls.
Figures
References
-
- Campsen J, Zimmerman MA, Trotter JF, et al. Sirolimus and liver transplantation: clinical implications for hepatocellular carcinoma. Expert Opinion on Pharmacotherapy. 2007;8(9):1275–1282. - PubMed
-
- Firpi RJ, Tran TT, Flores P, et al. Sirolimus-induced hyperlipidaemia in liver transplant recipients is not dose-dependent. Alimentary Pharmacology and Therapeutics. 2004;19(9):1033–1039. - PubMed
-
- Fisher A, Seguel JM, de la Torre AN, et al. Effect of sirolimus on infection incidence in liver transplant recipients. Liver Transplantation. 2004;10(2):193–198. - PubMed
-
- Kniepeiss D, Iberer F, Grasser B, Schaffellner S, Tscheliessnigg KH. Sirolimus and mycophenolate mofetil after liver transplantation. Transplant International. 2003;16(7):504–509. - PubMed
-
- McAlister VC, Peltekian KM, Malatjalian DA, et al. Orthotopic liver transplantation using low-dose tacrolimus and sirolimus. Liver Transplantation. 2001;7(8):701–708. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

