Links between hydrothermal environments, pyrophosphate, na(+), and early evolution
- PMID: 21461648
- PMCID: PMC3178022
- DOI: 10.1007/s11084-011-9235-4
Links between hydrothermal environments, pyrophosphate, na(+), and early evolution
Abstract
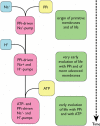
The discovery that photosynthetic bacterial membrane-bound inorganic pyrophosphatase (PPase) catalyzed light-induced phosphorylation of orthophosphate (Pi) to pyrophosphate (PPi) and the capability of PPi to drive energy requiring dark reactions supported PPi as a possible early alternative to ATP. Like the proton-pumping ATPase, the corresponding membrane-bound PPase also is a H(+)-pump, and like the Na(+)-pumping ATPase, it can be a Na(+)-pump, both in archaeal and bacterial membranes. We suggest that PPi and Na(+) transport preceded ATP and H(+) transport in association with geochemistry of the Earth at the time of the origin and early evolution of life. Life may have started in connection with early plate tectonic processes coupled to alkaline hydrothermal activity. A hydrothermal environment in which Na(+) is abundant exists in sediment-starved subduction zones, like the Mariana forearc in the W Pacific Ocean. It is considered to mimic the Archean Earth. The forearc pore fluids have a pH up to 12.6, a Na(+)-concentration of 0.7 mol/kg seawater. PPi could have been formed during early subduction of oceanic lithosphere by dehydration of protonated orthophosphates. A key to PPi formation in these geological environments is a low local activity of water.
Figures

References
-
- Abbona F, Franchini-Angela M. Crystallisation of calcium and magnesium phosphates from solutions of low concentration. J Cryst Growth. 1990;104:661–671. doi: 10.1016/0022-0248(90)90009-A. - DOI
-
- Alt JC, Teagle DAH. The uptake of carbon during alteration of ocean crust. Geochim Cosmochim Acta. 1999;63:1527–1535. doi: 10.1016/S0016-7037(99)00123-4. - DOI
-
- Alt JC, Shanks WC. Stable isotope compositions of serpentinite seamounts in the Mariana forearc: serpentinization processes, fluids sources and sulfur metasomatism. Earth Planet Sci Lett. 2006;242:272–285. doi: 10.1016/j.epsl.2005.11.063. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

