Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells
- PMID: 21461989
- PMCID: PMC3204002
- DOI: 10.1007/s00281-011-0266-8
Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells
Abstract
Pluripotent stem cells hold great promises for regenerative medicine. They might become useful as a universal source for a battery of new cell replacement therapies. Among the major concerns for the clinical application of stem cell-derived grafts are the risks of immune rejection and tumor formation. Pluripotency and tumorigenicity are closely linked features of pluripotent stem cells. However, the capacity to form teratomas or other tumors is not sufficiently described by inherited features of a stem cell line or a stem cell-derived graft. The tumorigenicity always depends on the inability of the recipient to reject the tumorigenic cells. This review summarizes recent data on the tumorigenicity of pluripotent stem cells in immunodeficient, syngeneic, allogeneic, and xenogeneic hosts. The effects of immunosuppressive treatment and cell differentiation are discussed. Different immune effector mechanisms appear to be involved in the rejection of undifferentiated and differentiated cell populations. Elements of the innate immune system, such as natural killer cells and the complement system, which are active also in syngeneic recipients, appear to preferentially reject undifferentiated cells. This effect could reduce the risk of tumor formation in immunocompetent recipients. Cell differentiation apparently increases susceptibility to rejection by the adaptive immune system in allogeneic hosts. The current data suggest that the immune system of the recipient has a major impact on the outcome of pluripotent stem cell transplantation, whether it is rejection, engraftment, or tumor development. This has to be considered when the results of experimental transplantation models are interpreted and even more when translation into clinics is planned.
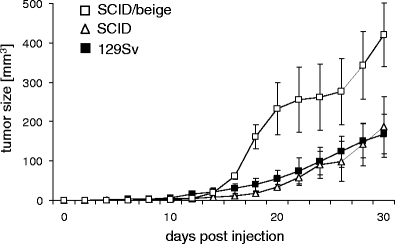
Figures

Similar articles
-
Multipotent adult germ-line stem cells, like other pluripotent stem cells, can be killed by cytotoxic T lymphocytes despite low expression of major histocompatibility complex class I molecules.Biol Direct. 2009 Aug 28;4:31. doi: 10.1186/1745-6150-4-31. Biol Direct. 2009. PMID: 19715575 Free PMC article.
-
Highly sensitive biosafety model for stem-cell-derived grafts.Cytotherapy. 2004;6(3):212-22. doi: 10.1080/14653240410006031. Cytotherapy. 2004. PMID: 15203978
-
Targeted Disruption of the β2-Microglobulin Gene Minimizes the Immunogenicity of Human Embryonic Stem Cells.Stem Cells Transl Med. 2015 Oct;4(10):1234-45. doi: 10.5966/sctm.2015-0049. Epub 2015 Aug 18. Stem Cells Transl Med. 2015. PMID: 26285657 Free PMC article.
-
Potential barriers to therapeutics utilizing pluripotent cell derivatives: intrinsic immunogenicity of in vitro maintained and matured populations.Semin Immunopathol. 2011 Nov;33(6):563-72. doi: 10.1007/s00281-011-0269-5. Epub 2011 Apr 11. Semin Immunopathol. 2011. PMID: 21479877 Review.
-
Immunogenicity of in vitro maintained and matured populations: potential barriers to engraftment of human pluripotent stem cell derivatives.Methods Mol Biol. 2013;1029:17-31. doi: 10.1007/978-1-62703-478-4_2. Methods Mol Biol. 2013. PMID: 23756939 Free PMC article. Review.
Cited by
-
Reduced immunogenicity of induced pluripotent stem cells derived from Sertoli cells.PLoS One. 2014 Aug 28;9(8):e106110. doi: 10.1371/journal.pone.0106110. eCollection 2014. PLoS One. 2014. PMID: 25166861 Free PMC article.
-
Teratoma formation: a tool for monitoring pluripotency in stem cell research.Curr Protoc Stem Cell Biol. 2015 Feb 2;32:4A.8.1-4A.8.17. doi: 10.1002/9780470151808.sc04a08s32. Curr Protoc Stem Cell Biol. 2015. PMID: 25640819 Free PMC article.
-
Reprogramming human urine cells into intestinal organoids with long-term expansion ability and barrier function.Heliyon. 2024 Jun 27;10(13):e33736. doi: 10.1016/j.heliyon.2024.e33736. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040281 Free PMC article.
-
TGFβ Family Signaling Pathways in Pluripotent and Teratocarcinoma Stem Cells' Fate Decisions: Balancing Between Self-Renewal, Differentiation, and Cancer.Cells. 2019 Nov 23;8(12):1500. doi: 10.3390/cells8121500. Cells. 2019. PMID: 31771212 Free PMC article. Review.
-
Synergistic and Superimposed Effect of Bone Marrow-Derived Mesenchymal Stem Cells Combined with Fasudil in Experimental Autoimmune Encephalomyelitis.J Mol Neurosci. 2016 Dec;60(4):486-497. doi: 10.1007/s12031-016-0819-3. Epub 2016 Aug 30. J Mol Neurosci. 2016. PMID: 27573128
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

