Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study
- PMID: 21462215
- PMCID: PMC6652705
- DOI: 10.1002/clc.20846
Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study
Abstract
Background: Studies have suggested that micronutrient deficiency has some role in the progression of chronic heart failure (CHF).
Hypothesis: Oral supplementation with coenzyme Q(10) (CoQ(10)) and creatine may reduce mitochondrial dysfunction that contributes to impaired physical performance in CHF.
Methods: We conducted a randomized, double-blind, placebo-controlled trial to determine the effect of a mixture of water-soluble CoQ(10) (CoQ(10) terclatrate; Q-ter) and creatine on exercise tolerance and health-related quality of life. Exercise tolerance was measured as total work capacity (kg·m) and peak oxygen consumption (VO(2), mL/min/kg), both from a cardiopulmonary exercise test. Health-related quality of life was measured by the Sickness Impact Profile (SIP) in CHF secondary to left ventricular systolic dysfunction (left ventricular ejection fraction ≤ 35%). After baseline assessment, 67 patients with stable CHF were randomized to receive Q-ter 320 mg + creatine 340 mg (n = 35) or placebo (n = 32) once daily for 8 weeks.
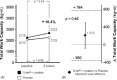
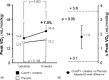
Results: At multivariate analysis, 8-week peak VO(2) was significantly higher in the active treatment group than in the placebo group (+1.8 ± 0.9 mL/min/kg, 95% CI: 0.1-3.6, P < 0.05). No untoward effects occurred in either group.
Conclusions: This study suggests that oral Q-ter and creatine, added to conventional drug therapy, exert some beneficial effect on physical performance in stable systolic CHF. Results may support the design of larger studies aimed at assessing the long-term effects of this treatment on functional status and harder outcomes.
© 2011 Wiley Periodicals, Inc.
Figures
Comment in
-
Coenzyme q10 and creatine in heart failure: micronutrients, macrobenefit?Clin Cardiol. 2011 Apr;34(4):196-7. doi: 10.1002/clc.20892. Clin Cardiol. 2011. PMID: 21462213 Free PMC article. No abstract available.
Similar articles
-
Coenzyme q10 and creatine in heart failure: micronutrients, macrobenefit?Clin Cardiol. 2011 Apr;34(4):196-7. doi: 10.1002/clc.20892. Clin Cardiol. 2011. PMID: 21462213 Free PMC article. No abstract available.
-
Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study.J Am Coll Cardiol. 2009 Sep 1;54(10):919-27. doi: 10.1016/j.jacc.2009.04.078. J Am Coll Cardiol. 2009. PMID: 19712802 Clinical Trial.
-
Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure.J Am Coll Cardiol. 1999 May;33(6):1549-52. doi: 10.1016/s0735-1097(99)00064-9. J Am Coll Cardiol. 1999. PMID: 10334422 Clinical Trial.
-
Efficacy and safety of coenzyme Q10 in heart failure: a meta-analysis of randomized controlled trials.BMC Cardiovasc Disord. 2024 Oct 26;24(1):592. doi: 10.1186/s12872-024-04232-z. BMC Cardiovasc Disord. 2024. PMID: 39462324 Free PMC article.
-
Ivabradine Improves Cardiac Function and Increases Exercise Capacity in Patients with Chronic Heart Failure.Int Heart J. 2019 Jul 27;60(4):899-909. doi: 10.1536/ihj.18-559. Epub 2019 Jul 12. Int Heart J. 2019. PMID: 31308326
Cited by
-
Effects of nutraceutical diet integration, with coenzyme Q10 (Q-Ter multicomposite) and creatine, on dyspnea, exercise tolerance, and quality of life in COPD patients with chronic respiratory failure.Multidiscip Respir Med. 2013 Jun 21;8(1):40. doi: 10.1186/2049-6958-8-40. Multidiscip Respir Med. 2013. PMID: 23800154 Free PMC article.
-
Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010-2020).Antioxidants (Basel). 2021 Aug 23;10(8):1325. doi: 10.3390/antiox10081325. Antioxidants (Basel). 2021. PMID: 34439573 Free PMC article. Review.
-
Role of Creatine in the Heart: Health and Disease.Nutrients. 2021 Apr 7;13(4):1215. doi: 10.3390/nu13041215. Nutrients. 2021. PMID: 33917009 Free PMC article. Review.
-
A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells.PLoS One. 2012;7(3):e33712. doi: 10.1371/journal.pone.0033712. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22432044 Free PMC article.
-
Coenzyme q10 and creatine in heart failure: micronutrients, macrobenefit?Clin Cardiol. 2011 Apr;34(4):196-7. doi: 10.1002/clc.20892. Clin Cardiol. 2011. PMID: 21462213 Free PMC article. No abstract available.
References
-
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112: 1825–1852. - PubMed
-
- Lloyd‐Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. - PubMed
-
- Marchionni N, Di Bari M, Fumagalli S, et al. Variable effect of comorbidity on the association of chronic cardiac failure with disability in community‐dwelling older persons. Arch Gerontol Geriatr. 1996;23:283–292. - PubMed
-
- Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health‐related quality of life in patients with heart failure. J Card Fail. 2004;10:368–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

