Microfabrication and nanotechnology in stent design
- PMID: 21462356
- PMCID: PMC3480085
- DOI: 10.1002/wnan.123
Microfabrication and nanotechnology in stent design
Abstract
Intravascular stents were first introduced in the 1980s as an adjunct to primary angioplasty for management of early complications, including arterial dissection, or treatment of an inadequate technical outcome due to early elastic recoil of the atherosclerotic lesion. Despite the beneficial effects of stenting, persistent high rates of restenosis motivated the design of drug-eluting stents for delivery of agents to limit the proliferative and other inflammatory responses within the vascular wall that contribute to the development of a restenotic lesion. These strategies have yielded a significant reduction in the incidence of restenosis, but challenges remain, including incomplete repair of the endothelium at the site of vascular wall injury that may be associated with a late risk of thrombosis. A failure of vessel wall healing has been attributed primarily to the use of polymeric stent coatings, but the effects of the eluted drug and other material properties or design features of the stent cannot be excluded. Improvements in stent microfabrication, as well as the introduction of alternative materials may help to address those limitations that inhibit stent performance. This review describes the application of novel microfabrication processes and the evolution of new nanotechnologies that hold significant promise in eliminating existing shortcomings of current stent platforms.
Copyright © 2011 John Wiley & Sons, Inc.
Figures
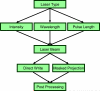





Similar articles
-
Drug-eluting stents.Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review.
-
Intravascular ultrasound to guide percutaneous coronary interventions: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(12):1-97. Epub 2006 Apr 1. Ont Health Technol Assess Ser. 2006. PMID: 23074482 Free PMC article.
-
The evolving impact of microfabrication and nanotechnology on stent design.J Vasc Surg. 2006 Dec;44(6):1363-8. doi: 10.1016/j.jvs.2006.08.046. J Vasc Surg. 2006. PMID: 17145446 Review.
-
Comparison between catheter-based delivery of paclitaxel after bare-metal stenting and drug-eluting stents in coronary artery disease patients at high risk for in-stent restenosis.Cardiovasc Revasc Med. 2017 Dec;18(8):596-600. doi: 10.1016/j.carrev.2017.05.018. Epub 2017 May 31. Cardiovasc Revasc Med. 2017. PMID: 28625402 Clinical Trial.
-
Perspectives of drug-eluting stents: the next revolution.Am J Cardiovasc Drugs. 2002;2(3):163-72. doi: 10.2165/00129784-200202030-00004. Am J Cardiovasc Drugs. 2002. PMID: 14727979 Review.
Cited by
-
An insight into biomimetic 4D printing.RSC Adv. 2019 Nov 22;9(65):38209-38226. doi: 10.1039/c9ra07342f. eCollection 2019 Nov 19. RSC Adv. 2019. PMID: 35541793 Free PMC article. Review.
-
Surface engineering at the nanoscale: A way forward to improve coronary stent efficacy.APL Bioeng. 2021 Jun 1;5(2):021508. doi: 10.1063/5.0037298. eCollection 2021 Jun. APL Bioeng. 2021. PMID: 34104846 Free PMC article. Review.
-
Balloon expandable coronary stent materials: a systematic review focused on clinical success.In Vitro Model. 2022 Jan 31;1(2):151-175. doi: 10.1007/s44164-022-00009-w. eCollection 2022 Apr. In Vitro Model. 2022. PMID: 39872801 Free PMC article. Review.
-
Advances in Fabrication Technologies for the Development of Next-Generation Cardiovascular Stents.J Funct Biomater. 2023 Nov 10;14(11):544. doi: 10.3390/jfb14110544. J Funct Biomater. 2023. PMID: 37998113 Free PMC article. Review.
-
Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis.Theranostics. 2014 Jan 8;4(2):175-200. doi: 10.7150/thno.7210. eCollection 2014. Theranostics. 2014. PMID: 24465275 Free PMC article. Review.
References
-
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. American Heart Journal. 2006;151(6):1260–4. - PubMed
-
- Doyle B, Rihal CS, O'Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007 Nov 20;116(21):2391–8. - PubMed
-
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006 Jul 4;48(1):193–202. - PubMed
-
- Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007 Feb 27;115(8):1051–8. - PubMed
-
- Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007 Mar 8;356(10):998–1008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

