Influenza virus assays based on virus-inducible reporter cell lines
- PMID: 21462401
- PMCID: PMC4940803
- DOI: 10.1111/j.1750-2659.2009.00095.x
Influenza virus assays based on virus-inducible reporter cell lines
Abstract
Background: Virus-inducible reporter genes have been used as the basis of virus detection and quantitation assays for a number of viruses. A strategy for influenza A virus-induction of a reporter gene was recently described. In this report, we describe the extension of this strategy to influenza B virus, the generation of stable cell lines with influenza A and B virus-inducible reporter genes, and the use of these cells in various clinically relevant viral assays. Each of the cell lines described herein constitutively express an RNA transcript that contains a reporter gene coding region flanked by viral 5¢- and 3¢-untranslated regions (UTR) and therefore mimics an influenza virus genomic segment. Upon infection of the cells with influenza virus the virus-inducible reporter gene segment (VIRGS) is replicated and transcribed by the viral polymerase complex resulting in reporter gene expression.
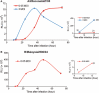
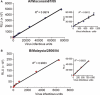
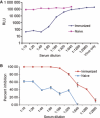
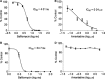
Findings: Reporter gene induction occurs after infection with a number of laboratory strains and clinical isolates of influenza virus including several H5N1 strains. The induction is dose-dependent and highly specific for influenza A or influenza B viruses.
Conclusions: These cell lines provide the basis of simple, rapid, and objective assays that involve virus quantitation such as determination of viral titer, assessment of antiviral susceptibility, and determination of antibody neutralization titer. These cell lines could be very useful for influenza virus researchers and vaccine manufacturers.
Figures

Similar articles
-
Establishment and characterization of a Madin-Darby canine kidney reporter cell line for influenza A virus assays.J Clin Microbiol. 2010 Jul;48(7):2515-23. doi: 10.1128/JCM.02286-09. Epub 2010 May 26. J Clin Microbiol. 2010. PMID: 20504984 Free PMC article.
-
Virus-inducible reporter genes as a tool for detecting and quantifying influenza A virus replication.J Virol Methods. 2005 Jun;126(1-2):13-20. doi: 10.1016/j.jviromet.2005.01.016. J Virol Methods. 2005. PMID: 15847914 Free PMC article.
-
Optimisations and Challenges Involved in the Creation of Various Bioluminescent and Fluorescent Influenza A Virus Strains for In Vitro and In Vivo Applications.PLoS One. 2015 Aug 4;10(8):e0133888. doi: 10.1371/journal.pone.0133888. eCollection 2015. PLoS One. 2015. PMID: 26241861 Free PMC article.
-
Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics.J Infect Dis. 2006 Nov 1;194 Suppl 2:S98-110. doi: 10.1086/507554. J Infect Dis. 2006. PMID: 17163396 Review.
-
Monitoring of viral susceptibility: new challenges with the development of influenza NA inhibitors.Rev Med Virol. 2000 Jan-Feb;10(1):45-55. doi: 10.1002/(sici)1099-1654(200001/02)10:1<45::aid-rmv265>3.0.co;2-r. Rev Med Virol. 2000. PMID: 10654004 Review.
Cited by
-
A Comprehensive Roadmap Towards the Generation of an Influenza B Reporter Assay Using a Single DNA Polymerase-Based Cloning of the Reporter RNA Construct.Front Microbiol. 2022 May 25;13:868367. doi: 10.3389/fmicb.2022.868367. eCollection 2022. Front Microbiol. 2022. PMID: 35694292 Free PMC article.
-
Replication-incompetent influenza A viruses that stably express a foreign gene.J Gen Virol. 2011 Dec;92(Pt 12):2879-2888. doi: 10.1099/vir.0.037648-0. Epub 2011 Aug 31. J Gen Virol. 2011. PMID: 21880840 Free PMC article.
-
High-throughput screening for identification of influenza a inhibitors using a cell-based immunofluorescence assay.Antiviral Res. 2025 Aug;240:106209. doi: 10.1016/j.antiviral.2025.106209. Epub 2025 Jun 6. Antiviral Res. 2025. PMID: 40484321
-
High-Throughput cell-based immunofluorescence assays against influenza.SLAS Discov. 2024 Jan;29(1):66-76. doi: 10.1016/j.slasd.2023.10.008. Epub 2023 Nov 2. SLAS Discov. 2024. PMID: 37925159 Free PMC article.
-
Cell Cultures for Virology: Usability, Advantages, and Prospects.Int J Mol Sci. 2020 Oct 27;21(21):7978. doi: 10.3390/ijms21217978. Int J Mol Sci. 2020. PMID: 33121109 Free PMC article. Review.
References
-
- Molinari NA, Ortega‐Sanchez IR, Messonnier ML et al. The annual impacrt of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096. - PubMed
-
- Update: influenza activity ‐ United States, September 28, 2008 – January 31, 2009. MMWR 2009; 58:115–119. - PubMed
-
- Kreijtz JH, Osterhaus AD, Rimmelzwaan GF. Vaccination strategies and vaccine formulations for epidemic and pandemic influenza control. Hum Vaccin 2009; 5(3):126–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

