Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy
- PMID: 21463133
- PMCID: PMC3107373
- DOI: 10.1517/14712598.2011.573476
Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy
Abstract
Introduction: Chimeric antigen receptors (CARs) usually combine the antigen binding site of a monoclonal antibody with the signal activating machinery of a T cell, freeing antigen recognition from MHC restriction and thus breaking one of the barriers to more widespread application of cellular therapy. Similar to treatment strategies employing monoclonal antibodies, T cells expressing CARs are highly targeted, but additionally offer the potential benefits of active trafficking to tumor sites, in vivo expansion and long-term persistence. Furthermore, gene transfer allows the introduction of countermeasures to tumor immune evasion and of safety mechanisms.
Areas covered: The basic structure of so-called first and later generation CARs and their potential advantages over other immune therapy systems. How these molecules can be grafted into immune cells (including retroviral and non-retroviral transduction methods) and strategies to improve the in vivo persistence and function of immune cells expressing CARs. Examples of tumor-associated antigens that have been targeted in preclinical models and clinical experience with these modified cells. Safety issues surrounding CAR gene transfer into T cells and potential solutions to them.
Expert opinion: Because of recent advances in immunology, genetics and cell processing, CAR-modified T cells will likely play an increasing role in the cellular therapy of cancer, chronic infections and autoimmune disorders.
Figures


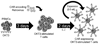

References
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990 Feb 1;75(3):555–562. - PubMed
-
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990 Dec 15;76(12):2462–2465. - PubMed
-
- Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994 Apr 28;330(17):1185–1191. - PubMed
-
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995 Jan 7;345(8941):9–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
