Activation of F-actin binding capacity of ezrin: synergism of PIP₂ interaction and phosphorylation
- PMID: 21463584
- PMCID: PMC3072610
- DOI: 10.1016/j.bpj.2011.02.039
Activation of F-actin binding capacity of ezrin: synergism of PIP₂ interaction and phosphorylation
Abstract
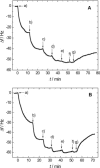
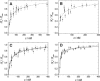
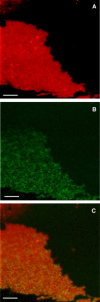
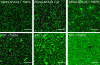
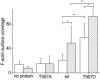
Ezrin is a membrane-cytoskeleton linker protein that can bind F-actin in its active conformation. Several means of regulation of ezrin's activity have been described including phosphorylation of Thr-567 and binding of L-α-phosphatidylinositol-4,5-bisphosphate (PIP(2)). However, the relative contributions of these events toward activation of the protein and their potential interdependence are not known. We developed an assay based on solid-supported membranes, to which different ezrin mutants (ezrin T567A (inactive mutant), wild-type, and T567D (active pseudophosphorylated mutant)) were bound, that enabled us to analyze the influence of phosphorylation and PIP(2) binding on ezrin's activation state in vitro. The lipid bilayers employed contained either DOGS-NTA-Ni to bind the proteins via an N-terminal His-tag, or PIP(2), to which ezrin binds via specific binding sites located in the N-terminal region of the protein. Quantitative analysis of the binding behavior of all three proteins to the two different receptor lipids revealed that all three bind with high affinity and specificity to the two receptor lipids. Fluorescence microscopy on ezrin-decorated solid-supported membranes showed that, dependent on the mode of binding and the phosphorylation state, ezrin is capable of binding actin filaments. A clear synergism between phosphorylation and the receptor lipid PIP(2) was observed, suggesting a conformational switch from the dormant to the active, F-actin binding state by recognition of PIP(2), which is enhanced by the phosphorylation.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

