Regulatory light chain phosphorylation and N-terminal extension increase cross-bridge binding and power output in Drosophila at in vivo myofilament lattice spacing
- PMID: 21463587
- PMCID: PMC3072621
- DOI: 10.1016/j.bpj.2011.02.028
Regulatory light chain phosphorylation and N-terminal extension increase cross-bridge binding and power output in Drosophila at in vivo myofilament lattice spacing
Abstract
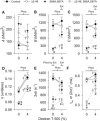
The N-terminal extension and phosphorylation of the myosin regulatory light chain (RLC) independently improve Drosophila melanogaster flight performance. Here we examine the functional and structural role of the RLC in chemically skinned fibers at various thick and thin filament lattice spacings from four transgenic Drosophila lines: rescued null or control (Dmlc2(+)), truncated N-terminal extension (Dmlc2(Δ2-46)), disrupted myosin light chain kinase phosphorylation sites (Dmlc2(S66A,S67A)), and dual mutant (Dmlc2(Δ2-46; S66A,S67A)). The N-terminal extension truncation and phosphorylation sites disruption mutations decreased oscillatory power output and the frequency of maximum power output in maximally Ca(2+)-activated fibers compressed to near in vivo inter-thick filament spacing, with the phosphorylation sites disruption mutation having a larger affect. The diminished power output parameters with the N-terminal extension truncation and phosphorylation sites disruption mutations were due to the reduction of the number of strongly-bound cross-bridges and rate of myosin force production, with the larger parameter reductions in the phosphorylation sites disruption mutation additionally related to reduced myosin attachment time. The phosphorylation and N-terminal extension-dependent boost in cross-bridge kinetics corroborates previous structural data, which indicate these RLC attributes play a complementary role in moving and orienting myosin heads toward actin target sites, thereby increasing fiber and whole fly power generation.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

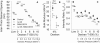
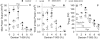

References
-
- Trybus K.M., Lowey S. Conformational states of smooth muscle myosin. Effects of light chain phosphorylation and ionic strength. J. Biol. Chem. 1984;259:8564–8571. - PubMed
-
- Sweeney H.L., Bowman B.F., Stull J.T. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am. J. Physiol. 1993;264:C1085–C1095. - PubMed
-
- Szczesna D., Zhao J., Potter J.D. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J. Appl. Physiol. 2002;92:1661–1670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

