The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians
- PMID: 21463979
- PMCID: PMC3237349
- DOI: 10.1289/ehp.1002956
The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians
Abstract
Background: Contaminants have been implicated in declines of amphibians, a taxon with vital systems similar to those of humans. However, many chemicals have not been thoroughly tested on amphibians or do not directly kill them.
Objective: Our goal in this study was to quantify amphibian responses to chlorothalonil, the most commonly used synthetic fungicide in the United States.
Methods: We reared Rana sphenocephala (southern leopard frog) and Osteopilus septentrionalis (Cuban treefrog) in outdoor mesocosms with or without 1 time (1×) and 2 times (2×) the expected environmental concentration (EEC) of chlorothalonil (~ 164 μg/L). We also conducted two dose-response experiments on O. septentrionalis, Hyla squirella (squirrel treefrog), Hyla cinerea (green treefrog), and R. sphenocephala and evaluated the effects of chlorothalonil on the stress hormone corticosterone.
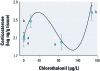
Results: For both species in the mesocosm experiment, the 1× and 2× EEC treatments were associated with > 87% and 100% mortality, respectively. In the laboratory experiments, the approximate EEC caused 100% mortality of all species within 24 hr; 82 μg/L killed 100% of R. sphenocephala, and 0.0164 μg/L caused significant tadpole mortality of R. sphenocephala and H. cinerea. Three species showed a nonmonotonic dose response, with low and high concentrations causing significantly greater mortality than did intermediate concentrations or control treatments. For O. septentrionalis, corticosterone exhibited a similar nonmonotonic dose response and chlorothalonil concentration was inversely associated with liver tissue and immune cell densities (< 16.4 μg/L).
Conclusions: Chlorothalonil killed nearly every amphibian at the approximate EEC; at concentrations to which humans are commonly exposed, it increased mortality and was associated with elevated corticosterone levels and changes in immune cells. Future studies should directly quantify the effects of chlorothalonil on amphibian populations and human health.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures

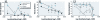
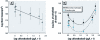
References
-
- Baier-Anderson C, Anderson RS. The effects of chlorothalonil on oyster hemocyte activation: phagocytosis, reduced pyridine nucleotides, and reactive oxygen species production. Environ Res. 2000;83:72–78. - PubMed
-
- Belden J, McMurry S, Smith L, Reilley P. Acute toxicity of fungicide formulations to amphibians at environmentally relevant concentrations. Environ Toxicol Chem. 2010;29:2477–2480. - PubMed
-
- Burger W, Burge M. Quantifying Stained Liver Tissue. 2009. Available: http://rsb.info.nih.gov/ij/docs/examples/stained-sections4/ [accessed 24 June 2011]
-
- Caux PY, Kent RA, Fan GT, Stephenson GL. Environmental fate and effects of chlorothalonil: a Canadian perspective. Crit Rev Environ Sci Technol. 1996;26:45–93.
-
- Cohen LM, Neimark HL, Everland LK. Schistosoma mansoni: response to cercariae to a thermal gradient. J Parasitol. 1980;66:362–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
