Convection-enhanced delivery of topotecan into a PDGF-driven model of glioblastoma prolongs survival and ablates both tumor-initiating cells and recruited glial progenitors
- PMID: 21464045
- PMCID: PMC3113406
- DOI: 10.1158/0008-5472.CAN-10-0906
Convection-enhanced delivery of topotecan into a PDGF-driven model of glioblastoma prolongs survival and ablates both tumor-initiating cells and recruited glial progenitors
Abstract
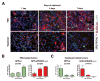
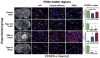
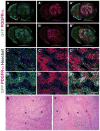
The contribution of microenvironment to tumor growth has important implications for optimizing chemotherapeutic response and understanding the biology of recurrent tumors. In this study, we tested the effects of locally administered topotecan on a rat model of glioblastoma that is induced by intracerebral injection of PDGF (platelet-derived growth factor)-IRES (internal ribosome entry site)-GFP (green fluorescent protein)-expressing retrovirus, treated the tumors by convection-enhanced delivery (CED) of topotecan (136 μmol/L) for 1, 4, or 7 days, and then characterized the effects on both the retrovirus-transformed tumor cells (GFP(+) cells) as well as the uninfected glial progenitor cells (GFP(-) cells) that are recruited to the tumor. Topotecan treatment reduced GFP(+) cells about 10-fold and recruited progenitors by about 80-fold while providing a significant survival advantage that improved with greater treatment duration. Regions of glial progenitor ablation occurred corresponding to the anatomic distribution of topotecan as predicted by MRI of a surrogate tracer. Histopathologic changes in recurrent tumors point to a decrease in recruitment, most likely due to the chemotherapeutic ablation of the recruitable progenitor pool.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures



References
-
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. - PubMed
-
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. - PubMed
-
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

