Nkx2-5 represses Gata1 gene expression and modulates the cellular fate of cardiac progenitors during embryogenesis
- PMID: 21464046
- PMCID: PMC3110259
- DOI: 10.1161/CIRCULATIONAHA.110.008185
Nkx2-5 represses Gata1 gene expression and modulates the cellular fate of cardiac progenitors during embryogenesis
Abstract
Background: Recent studies suggest that the hematopoietic and cardiac lineages have close ontogenic origins, and that an early mesodermal cell population has the potential to differentiate into both lineages. Studies also suggest that specification of these lineages is inversely regulated. However, the transcriptional networks that govern the cell fate specification of these progenitors are incompletely defined.
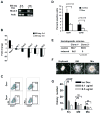
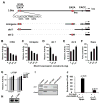
Methods and results: Here, we show that Nkx2-5 regulates the hematopoietic/erythroid fate of the mesoderm precursors early during cardiac morphogenesis. Using transgenic technologies to isolate Nkx2-5 expressing cells, we observed an induction of the erythroid molecular program, including Gata1, in the Nkx2-5-null embryos. We further observed that overexpression of Nkx2-5 with an Nkx2-5-inducible embryonic stem cell system significantly repressed Gata1 gene expression and suppressed the hematopoietic/erythroid potential, but not the endothelial potential, of the embryonic stem cells. This suppression was cell-autonomous, and was partially rescued by overexpressing Gata1. In addition, we demonstrated that Nkx2-5 binds to the Gata1 gene enhancer and represses the transcriptional activity of the Gata1 gene.
Conclusions: Our results demonstrate that the hematopoietic/erythroid cell fate is suppressed via Nkx2-5 during mesodermal fate determination, and that the Gata1 gene is one of the targets that are suppressed by Nkx2-5.
Figures




References
-
- Cossu G, Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. - PubMed
-
- Mikkola HK, Orkin SH. The search for the hemangioblast. J Hematother Stem Cell Res. 2002;11:9–17. - PubMed
-
- Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–1104. - PubMed
-
- Masino AM, Gallardo TD, Wilcox CA, Olson EN, Williams RS, Garry DJ. Transcriptional regulation of cardiac progenitor cell populations. Circ Res. 2004;95:389–397. - PubMed
-
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

