Potential of recombinant opa proteins as vaccine candidates against hyperinvasive meningococci
- PMID: 21464082
- PMCID: PMC3191958
- DOI: 10.1128/IAI.01338-10
Potential of recombinant opa proteins as vaccine candidates against hyperinvasive meningococci
Abstract
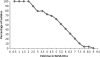
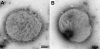
Neisseria meningitidis causes half a million cases of septicemia and meningitis globally each year. The opacity (Opa) integral outer membrane proteins from N. meningitidis are polymorphic and highly immunogenic. Particular combinations of Opa proteins are associated with the hyperinvasive meningococcal lineages that have caused the majority of serogroup B and C meningococcal disease in industrialized countries over the last 60 years. For the first time, this genetic structuring of a diverse outer membrane protein family has been used to select a novel combination of representative antigens for immunogenicity testing. Fourteen recombinant Opa variants were produced and used in murine immunizations inducing an increase in specific antimeningococcal total IgG levels. All 14 Opa proteins elicited bactericidal antibodies against at least one hyperinvasive meningococcal isolate, and most isolates from each hyperinvasive lineage were killed by at least one Opa antiserum at a titer of 1:16 or greater. Cross-reactive bactericidal antibody responses were observed among clonal complexes. A theoretical coverage of 90% can be achieved by using a particular combination of 6 Opa proteins against an isolate collection of 227 recent United Kingdom disease cases. This study indicates the potential of Opa proteins to provide broad coverage against multiple meningococcal hyperinvasive lineages.
Figures


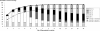
References
-
- Belland R. J., Morrison S. G., Carlson J. H., Hogan D. M. 1997. Promoter strength influences phase variation of neisserial opa genes. Mol. Microbiol. 23:123–135 - PubMed
-
- Borrow R., et al. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 24:5093–5107 - PubMed
-
- Boulton I. C., Gray-Owen S. D. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229–236 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

