Discovery of Salmonella virulence factors translocated via outer membrane vesicles to murine macrophages
- PMID: 21464085
- PMCID: PMC3125828
- DOI: 10.1128/IAI.01277-10
Discovery of Salmonella virulence factors translocated via outer membrane vesicles to murine macrophages
Abstract
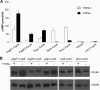
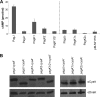
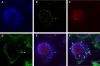
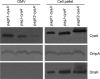
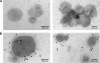
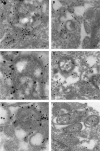
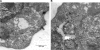
Salmonella enterica serovar Typhimurium, an intracellular pathogen and leading cause of food-borne illness, encodes a plethora of virulence effectors. Salmonella virulence factors are translocated into host cells and manipulate host cellular activities, providing a more hospitable environment for bacterial proliferation. In this study, we report a new set of virulence factors that is translocated into the host cytoplasm via bacterial outer membrane vesicles (OMV). PagK (or PagK1), PagJ, and STM2585A (or PagK2) are small proteins composed of ∼70 amino acids and have high sequence homology to each other (>85% identity). Salmonella lacking all three homologues was attenuated for virulence in a mouse infection model, suggesting at least partial functional redundancy among the homologues. While each homologue was translocated into the macrophage cytoplasm, their translocation was independent of all three Salmonella gene-encoded type III secretion systems (T3SSs)-Salmonella pathogenicity island 1 (SPI-1) T3SS, SPI-2 T3SS, and the flagellar system. Selected methods, including direct microscopy, demonstrated that the PagK-homologous proteins were secreted through OMV, which were enriched with lipopolysaccharide (LPS) and outer membrane proteins. Vesicles produced by intracellular bacteria also contained lysosome-associated membrane protein 1 (LAMP1), suggesting the possibility of OMV convergence with host cellular components during intracellular trafficking. This study identified novel Salmonella virulence factors secreted via OMV and demonstrated that OMV can function as a vehicle to transfer virulence determinants to the cytoplasm of the infected host cell.
Figures
References
-
- Amano A., Takeuchi H., Furuta N. 2010. Outer membrane vesicles function as offensive weapons in host-parasite interactions. Microbes Infect. 12:791–798 - PubMed
-
- Asensio C. J., et al. 2011. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29:1649–1656 - PubMed
-
- Ayala B. P., et al. 2001. The pilus-induced Ca2+ flux triggers lysosome exocytosis and increases the amount of Lamp1 accessible to Neisseria IgA1 protease. Cell. Microbiol. 3:265–275 - PubMed
-
- Beatty W. L., et al. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1:235–247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

