Phenotypes in mTERT⁺/⁻ and mTERT⁻/⁻ mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase
- PMID: 21464209
- PMCID: PMC3133422
- DOI: 10.1128/MCB.05312-11
Phenotypes in mTERT⁺/⁻ and mTERT⁻/⁻ mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase
Abstract
Telomerase is essential for telomere length maintenance. Mutations in either of the two core components of telomerase, telomerase RNA (TR) or the catalytic protein component telomerase reverse transcriptase (TERT), cause the genetic disorders dyskeratosis congenita, pulmonary fibrosis, and other degenerative diseases. Overexpression of the TERT protein has been reported to have telomere length-independent roles, including regulation of the Wnt signaling pathway. To examine the phenotypes of TERT haploinsufficiency and determine whether loss of function of TERT has effects other than those associated with telomere shortening, we characterized both mTERT⁺/⁻ and mTERT⁻/⁻ mice on the CAST/EiJ genetic background. Phenotypic analysis showed a loss of tissue renewal capacity with progressive breeding of heterozygous mice that was indistinguishable from that of mTR-deficient mice. mTERT⁻/⁻ mice, from heterozygous mTERT⁺/⁻ mouse crosses, were born at the expected Mendelian ratio (26.5%; n = 1,080 pups), indicating no embryonic lethality of this genotype. We looked for, and failed to find, hallmarks of Wnt deficiency in various adult and embryonic tissues, including those of the lungs, kidneys, brain, and skeleton. Finally, mTERT⁻/⁻ cells showed wild-type levels of Wnt signaling in vitro. Thus, while TERT overexpression in some settings may activate the Wnt pathway, loss of function in a physiological setting has no apparent effects on Wnt signaling. Our results indicate that both TERT and TR are haploinsufficient and that their deficiency leads to telomere shortening, which limits tissue renewal. Our studies imply that hypomorphic loss-of-function alleles of hTERT and hTR should cause a similar disease spectrum in humans.
Figures

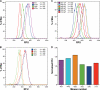

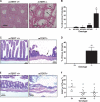
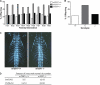


Comment in
-
Telomerase reverse transcriptase and Wnt signaling.Mol Cell Biol. 2011 Jun;31(12):2366-8. doi: 10.1128/MCB.05462-11. Epub 2011 May 2. Mol Cell Biol. 2011. PMID: 21536649 Free PMC article. No abstract available.
References
-
- Armanios M. Y., et al. 2007. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356: 1317–1326 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
