Plasma and intracellular tenofovir pharmacokinetics in the neonate (ANRS 12109 trial, step 2)
- PMID: 21464249
- PMCID: PMC3101430
- DOI: 10.1128/AAC.01377-10
Plasma and intracellular tenofovir pharmacokinetics in the neonate (ANRS 12109 trial, step 2)
Abstract
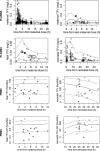
The objective of this study was to investigate for the first time tenofovir (TFV) pharmacokinetics in plasma and peripheral blood mononuclear cells (PBMCs) of the neonate. HIV-1-infected pregnant women received two tablets of tenofovir disoproxil fumarate (TDF; 300 mg) and emtricitabine (FTC; 200 mg) at onset of labor and then one tablet daily for 7 days postpartum. A single dose of 13 mg/kg of body weight of TDF was administered to 36 neonates within 12 h of life after the HIV-1-infected mothers had been administered two tablets of TDF-emtricitabine at delivery. A total of 626 samples collected within the 2 days after the drug administration were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and analyzed by a population approach. In the neonate, the median TFV plasma area under the curve and minimal and maximal concentrations, respectively, were 3.73 mg/liter · h and 0.076 and 0.29 mg/liter. In PBMCs, TFV concentrations were detectable in all fetuses, whereas tenofovir diphosphate (TFV-DP) was quantifiable in only two fetuses, suggesting a lag in appearance of TFV-DP. The median TFV-DP neonatal concentration was 146 fmol/10⁶ cells (interquartile range [IQR], 53 to 430 fmol/10⁶ cells); two neonates had very high TFV-DP concentrations (1,530 and 2963 fmol/10⁶ cells). The 13-mg/kg TDF dose given to neonates produced plasma TFV and intracellular active TFV-DP concentrations similar to those in adults. This dose should be given immediately after birth to reduce the delay before the active compound TFV-DP appears in cells.
Figures


References
-
- Anderson P. L., Kakuda T. N., Lichtenstein K. A. 2004. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 38:743–753 - PubMed
-
- Arrive E., et al. 2009. Tolerance and viral resistance after single-dose nevirapine with tenofovir and emtricitabine to prevent vertical transmission of HIV-1. AIDS 23:825–833 - PubMed
-
- Arrive E., et al. 2007. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int. J. Epidemiol. 36:1009–1021 - PubMed
-
- Benech H., et al. 2004. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 330:172–174 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

